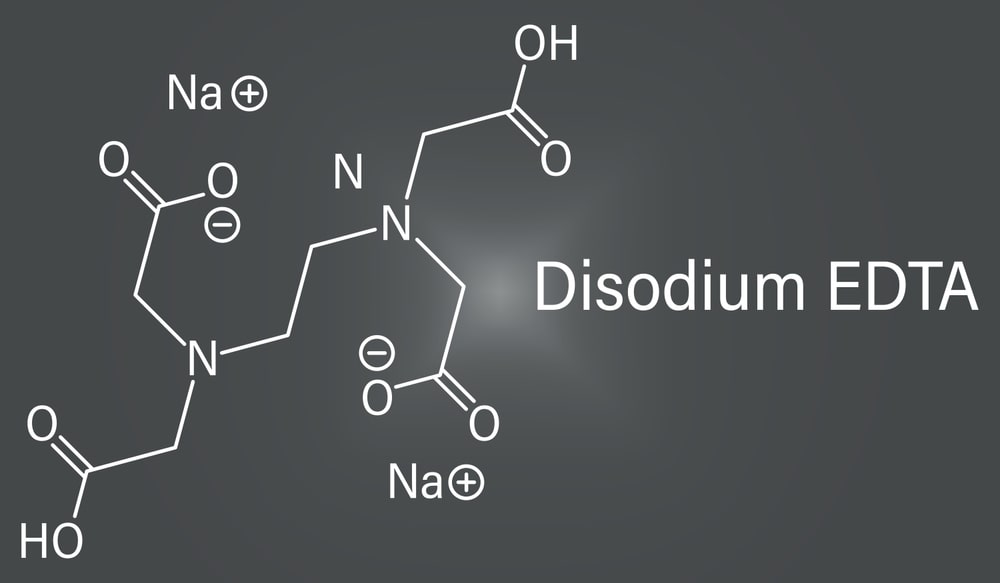
Disodium Edetate (0.1 M)
Molecular formula: C10H14N2Na2O8
Molecular weight: 372.24
Preparation: Dissolve 37.2 g of disodium edetate in sufficient water to produce 1000 mL.
Standardization: Weigh accurately about 0.8 g of granulated zinc, and dissolve by gentle warming in 12 mL of dilute hydrochloric acid and 0.1 mL of bromine water. Boil to remove excess bromine, cool, and add sufficient water to produce 200.0 mL. Pipette 20.0 mL of the resulting solution into a flask and neutralize with 2 M sodium hydroxide. Dilute to about 150 mL with water, add sufficient ammonia buffer pH 10.0 to dissolve the precipitate, and add 5 mL in excess. Add 50 mg of mordant black II mixture and titrate with the disodium edetate solution until the solution turns green.
Reaction:
Zn+2 + 2HCl → ZnCl2 + H2
ZnCl2 + Na2H2Y → [ZnH2Y] + 2NaCl
ZnCl2 + C10H14N2Na2O8 → [Zn– C10H14N2Na2O8] + 2NaCl
Factor Calculation:
1 mole of C10H14N2Na2O8 ≅ 1 mole of ZnCl2 ≅ 1 mole of Zn+2
1000 mL of 1M C10H14N2Na2O8 ≅ 65.38 g of Zn+2
1 mL of 0.1 M C10H14N2Na2O8 ≅ 0.006538 g of Zn+2
Make sure you also check our other amazing Article on : How do you prepare 0.1 M silver nitrate solution?