Synthesis and Reactions of Quinoline: Quinoline or 1-azanaphthalene is a colorless liquid with a sweetish odor and has a high boiling point of 237°C. It was first isolated in 1834 from coal tar. Quinoline and isoquinoline are benzopyridines, which are composed of a benzene ring fused to a pyridine ring. Quinoline is a weakly basic compound. Electron-releasing substituents at the 2 and 4 positions of quinoline increase the basicity. The electrophilic attack will take place at electron-rich C5 and C8 positions. Due to the presence of electronegative N-atom close to C2, the C2 and C4 positions are electron deficient. Hence, the nucleophilic attack takes place preferentially at the C2 and C4 positions.
Chemical Synthesis of Quinoline
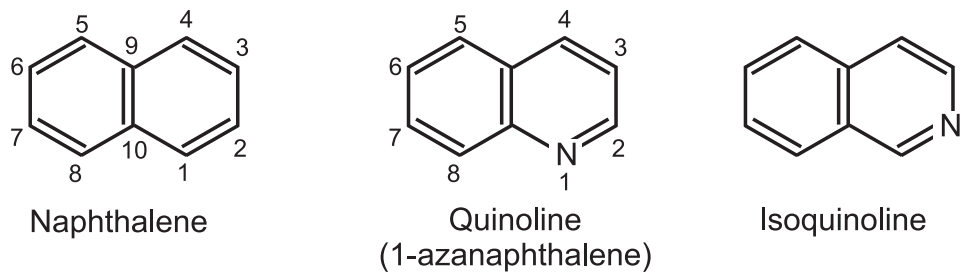
(1) Skraup synthesis: When aniline, concentrated sulfuric acid, glycerol, and a mild oxidizing agent are heated together, quinoline is formed. The reaction begins when glycerol is first dehydrated by concentrated sulfuric acid to acrolein. Aniline is then added begins to it to produce 1, 2-dihydroquinoline.
The 1, 2-dihydroquinoline is further oxidized to give quinoline. Aniline adds to acrolein through Michael’s addition to give aniline propanal (I). Substituted anilines give quinoline derivatives in which substituents appear in the benzene ring portion.
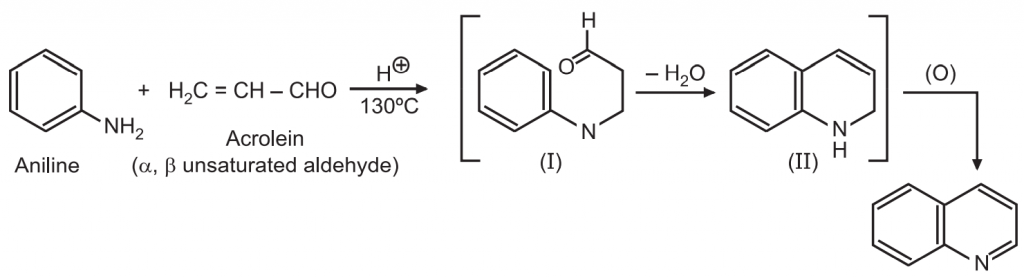
(2) Friedlander synthesis: When o-amino benzaldehyde or o-aminoacetophenone condenses with an aldehyde or ketone (which must contain an active α-methylene group) in alcoholic sodium hydroxide solution, it yields quinoline.
2-substituted quinoline derivatives are usually prepared by this method.
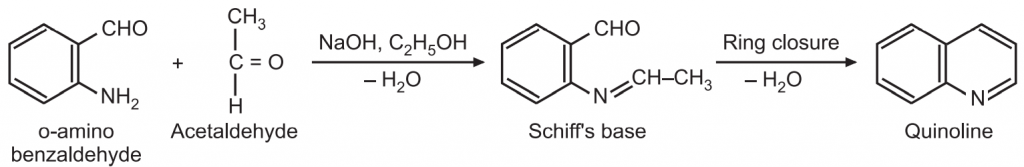
(3) Pfitzinger synthesis: In this method, isatin in the presence of a base, is converted to isotonic acid which is condensed with a ketone to give quinoline-4-carboxylic acid. The carboxylic acid group can be removed by pyrolysis with calcium oxide to give substituted quinolines.
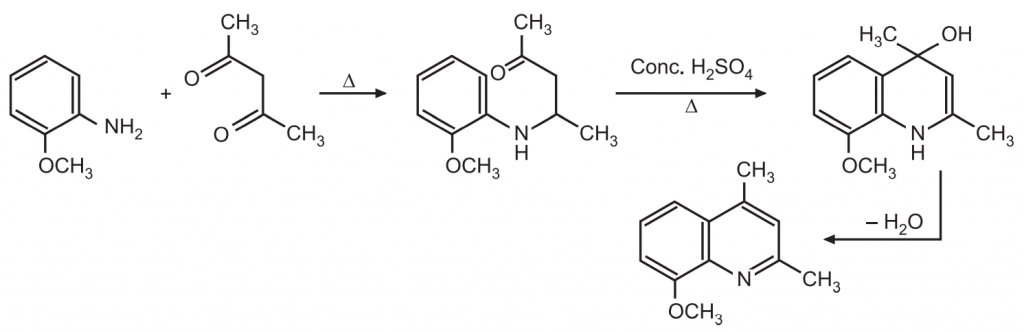
(4) Combes synthesis: Condensation of 1, 3-dicarbonyl compound with an arylamine gives a β-amino enone which undergoes cyclization with a loss of water to give quinoline.
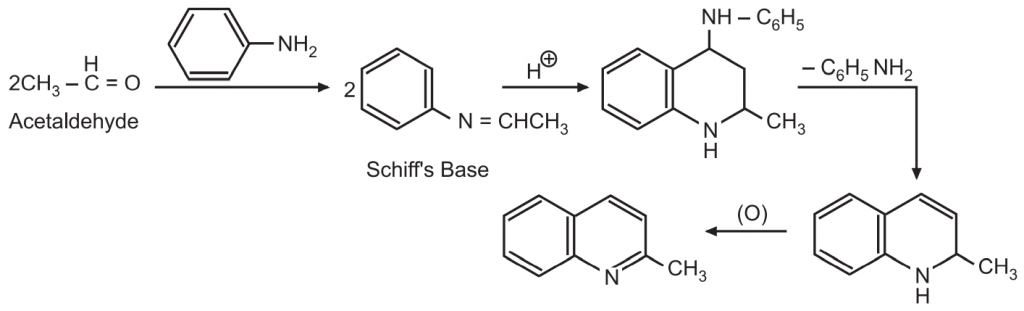
(5) Doebner Miller Synthesis: An aniline and two moles of acetaldehyde are heated in the presence of HCl to form Schiff’s base. Two molecules of this Schiff’s base condense to form a quinoline derivative.
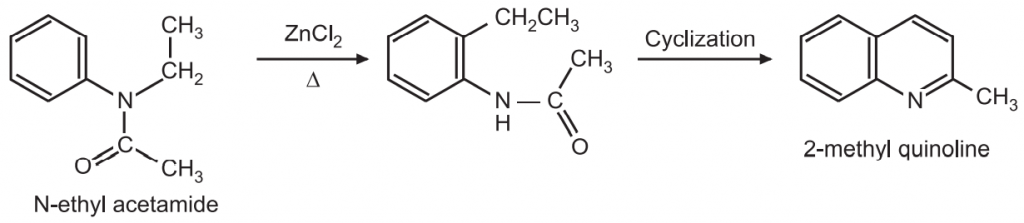
(6) Pictet Method:
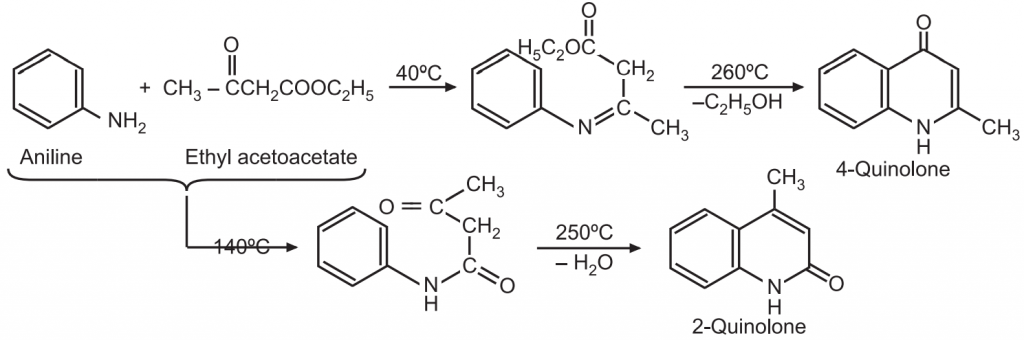
(7) Conrad – Limpach – Knorr synthesis: Anilines react with β-keto esters (e.g., ethyl acetoacetate) at a lower temperature to give a β-amino acrylate which upon cyclization gives a 4-quinolone. At high temperatures, β-ketoester anilides are formed which upon cyclization gives 2-quinolones.
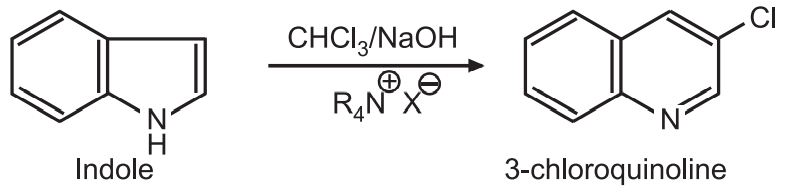
(8) Indole reacts with chloroform and sodium hydroxide in the presence of tetra-alkyl ammonium salt to give chloroquinoline.
Chemical Reactions of Quinoline
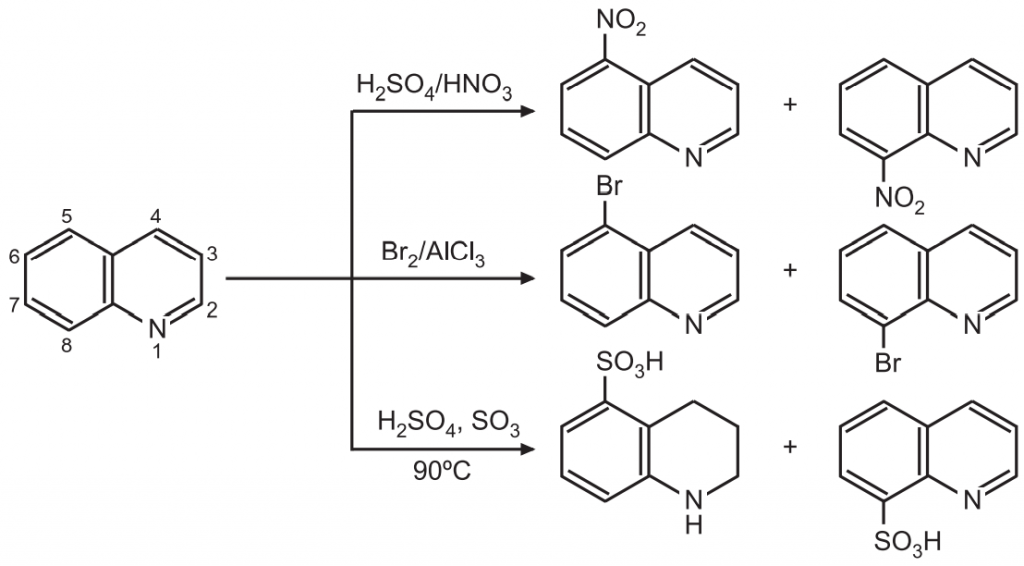
Quinoline is a weak base. The electron-rich N-atom of quinoline is the main site for attack by an electrophile. This N-atom deactivates the ring towards electrophilic attack. Hence, electrophilic substitution on quinoline rings requires vigorous reaction conditions. The electron-rich N-atom makes the pyridine ring (hetero ring) more π-electron deficient. Hence, electrophilic attack occurs at the benzo rather than more resistant hetero ring. While nucleophilic attack occurs at hetero ring rather than benzo-ring.
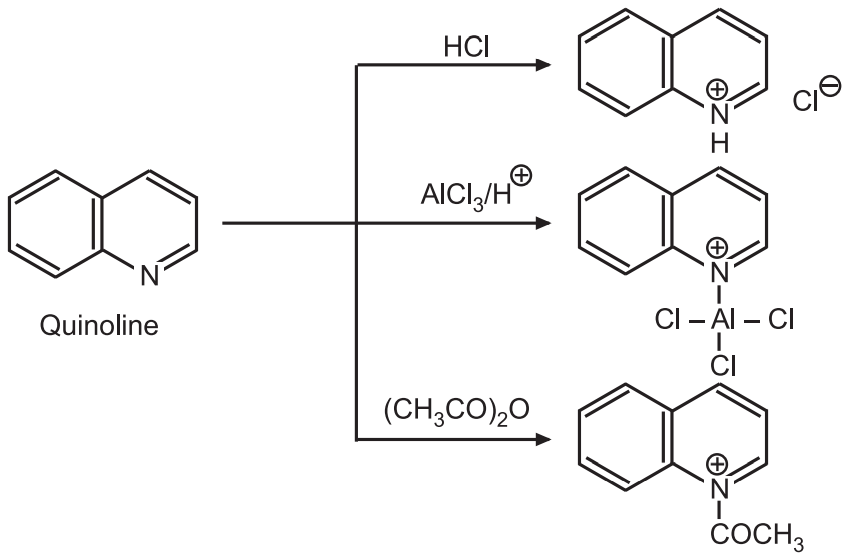
(1) Addition to nitrogen: The lone pair of electrons on the N-atom is available for extra bonding. When quinoline reacts with acids, metallic ions or acyl, sulfonyl, anhydrides, it forms quaternary salts.
(2) Electrophilic substitution: Electrophilic substitution preferably occurs at positions 5 and 8.
(a)
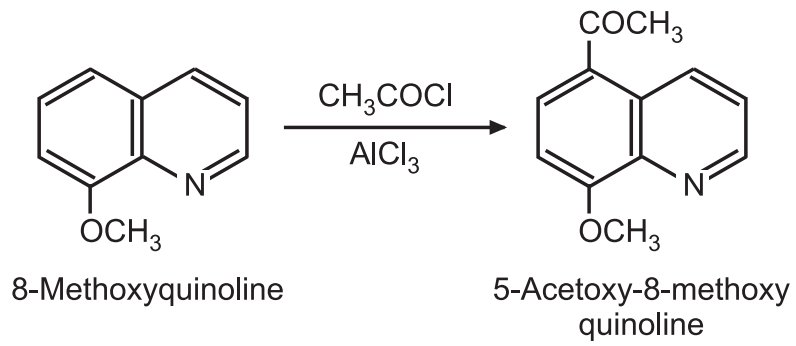
(b) Acylation and alkylation: The quinoline ring is already deactivated by the N-atom. Hence, alkylation and acylation occur only in those rings which have strong electron-donating substituents.
(3) Nucleophilic substitution: Quinoline readily gives a nucleophilic reaction. This reaction preferably occurs at position 2. If position 2 is already occupied then an attack may occur at position 4. For example,
(a)
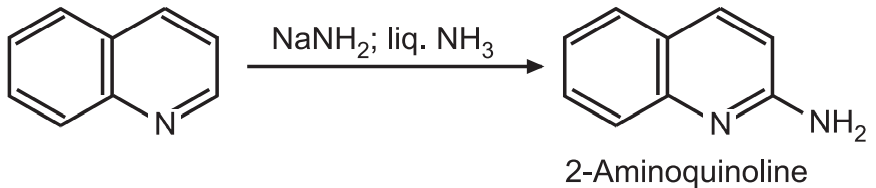
(b) Quinoline can be alkylated using organolithium reagents.
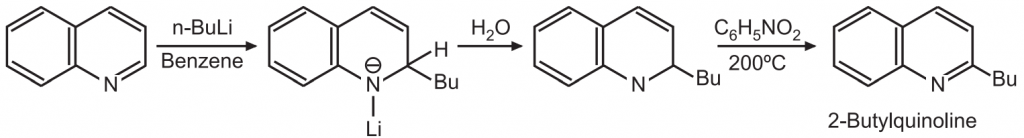
(c) Treatment with Grignard reagent results in the formation of substituted 1, 2- dihydroquinoline.
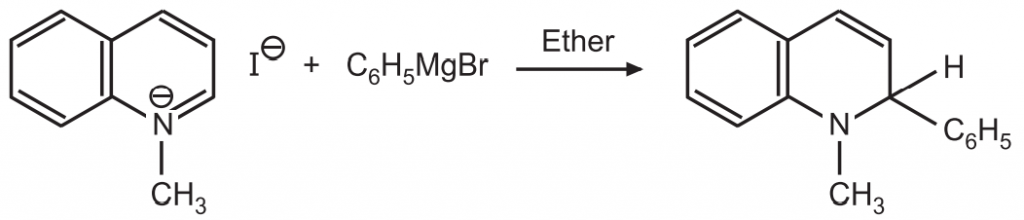
(d) Nucleophiles such as –NH2 or – OCH3 may attack the quinoline ring by displacing the halogen atom. Examples:
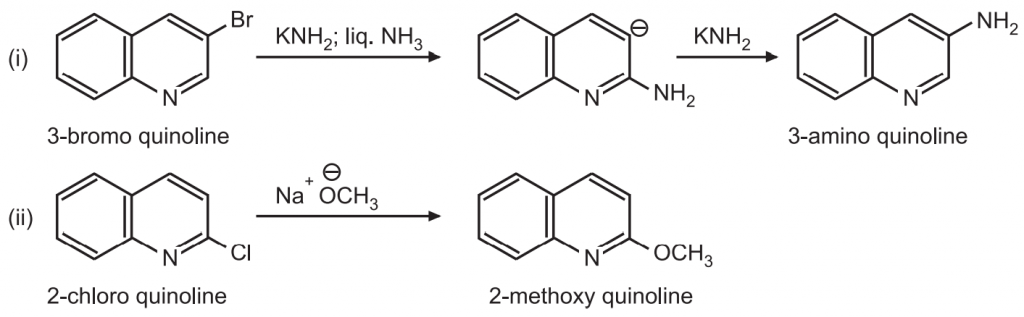
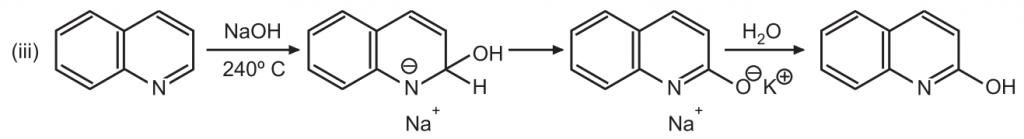
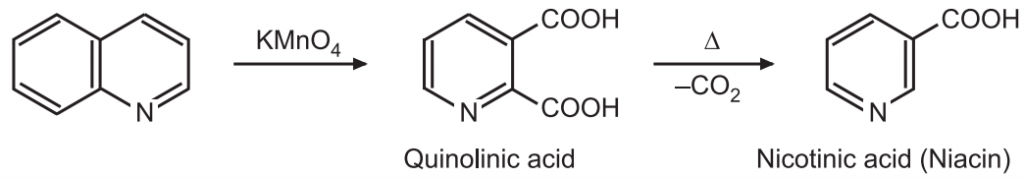
(4) Oxidation: Quinoline is resistant to oxidation. Under vigorous conditions, the pyridine ring remains intact while the benzene ring is opened up on treatment with alkaline KMnO4.
(5) Reduction: Quinoline is reduced to 1, 2, 3, 4 – tetrahydroquinoline using catalytic hydrogenation in methanol.
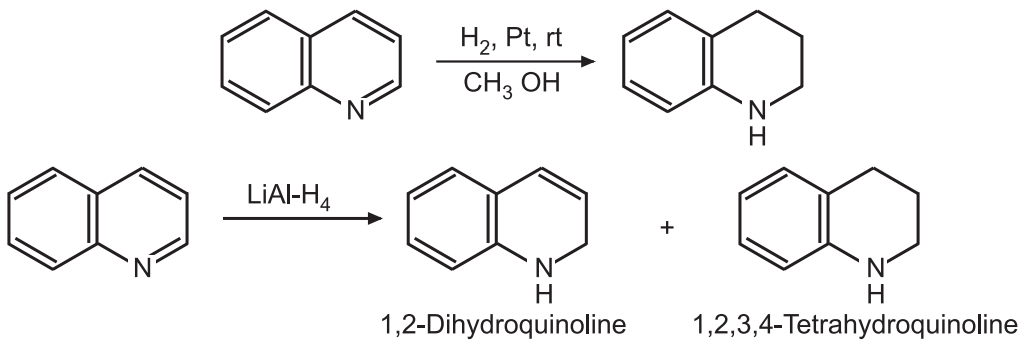
Lithium in liquid ammonia can produce, 1, 4 – dihydroquinoline under certain conditions. In an acid medium, the benzene ring can be selectively reduced.
Applications in Drug Synthesis
Quinoline is a core component in the structure of chloroquine (antimalarial), papaverine (smooth muscle relaxant), quinapril (antihypertensive), Singulair (anti-asthma), hydroxychloroquine (antimalarial), narcotine (depressant), emetine (emetic: vomiting inducer), dimethisoquin (anesthetic), etc.
Make sure you also check our other amazing Article on : Synthesis and Reactions of Thiazole
