Assay of Calcium Gluconate by Complexometry
Aim: To perform the assay of calcium gluconate by complexometry.
Requirement:
- Apparatus: Beaker, Funnel, Pipette, Burrette.
- Chemicals: Magnesium sulphate, Strong ammonia solution, Calcium gluconate.
Monograph:
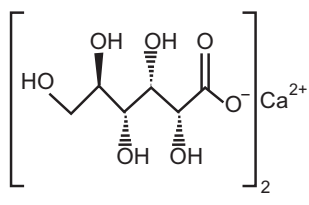
- C12H22CaO14H2O
- Molecular weight 448.4
- Calcium gluconate is a calcium o-gluconate monohydrate.
- Calcium gluconate contains not less than 98.5 per cent and not more than 102.0 per cent of C12H22CaO14H2O.
- Category: Calcium replenisher.
- Dose: Orally, up to 15 g daily, in divided doses. By intramuscular or slow intravenous injection, 1 to 2 g (500 mg of calcium gluconate is approximately equivalent to 2.3 mmol of Ca2+).
- Description: A white, crystalline powder or granules.
Procedure:
(A) Standardisation of Disodium EDTA: Preparation of disodium EDTA (0.1 M): Dissolve 37.2 g of disodium EDTA in sufficient water to produce 1000 ml.
Procedure: Weigh accurately about 0.8 g of granulated zinc, dissolve by gentle warming in 12 ml of dilute hydrochloric acid and add 0.1 ml of bromine water. Boil to remove excess bromine, cool and add sufficient water to produce 200.0 ml. Pipette out 20.0 ml of the resulting solution into a flask and nearly neutralize with 2 M sodium hydroxide. Dilute to about 150 ml with water, add sufficient ammonium buffer of pH 10.0 to dissolve the precipitate and add 5 ml in excess. Add 50 mg of Mordant Black II mixture and titrate with disodium EDTA solution until the solution turns green.
1 ml of 0.1 M disodium EDTA is equivalent to 0.000654 g of Zn.
(B) Assay: Weigh accurately about 0.5 g and dissolve in 50 ml of warm water; cool, add 5.0 ml of 0.05 M magnesium sulphate and 10 ml of strong ammonia solution and titrate with 0.05 M disodium EDTA using mordant black II mixture as an indicator. From the volume of 0.05 M, disodium EDTA is required to subtract the volume of magnesium sulphate solution added.
1 ml of the remainder of 0.05 M disodium EDTA is equivalent to 0.02242 g of C12H22CaO14H2O.
Observation table: (Standardization)
| Sr. No. | Parameters | Reading |
| 1. | Burette solution | Disodium EDTA |
| 2. | Conical flask solution | Ammonium buffer, Granulated zinc |
| 3. | Indicator | Mordant Black II |
| 4. | Endpoint | Green |
| Burette reading B.R (ml) | Trial 1 | Trial 2 | Trial 3 | Average (BR) |
Observation table: (Assay)
| Sr. No. | Parameters | Reading |
| 1. | Burette solution | Disodium edetate |
| 2. | Conical flask solution | Magnesium sulphate, Strong ammonia solution |
| 3. | Indicator | Mordant Black II |
| 4. | Endpoint | Deep blue |
| Burette reading B.R (ml) | Trial 1 | Trial 2 | Trial 3 | Average (BR) |
Result: The % purity of calcium gluconate is …….
Make sure you also check our other amazing Article on : Complexometric Titration
