INTRODUCTION
Table of Contents
Bioassay are procedures by which the potency or the nature of the substance is estimated by studying its effects on living matter. The basic principle of such assays is to compare how much of a sample being tested produces the same biological
effect as a given quantity of a standard preparation. They are generally carried out by using either intact animals; as in the case of bio-assay of insulin by using rabbits or mice, bio-assay of digitalis by using guinea pigs or pigeons; or isolated animal organs or tissues as in the case of bio-assay of histamine by using guinea pig ileum, bio-assay of acetylcholine by using frog’s rectus abdominous muscle preparation.
Bioassay can be defined as a procedure for determining the quantitative relationship between the dose of a drug and the magnitude of biological response it evokes; or the determination of the potency or concentration of a biologically active drug of physical, chemical, or biological origin by using a biological indicator. The biological indicator could be a whole animal like a frog, mouse, rat, guinea pig, cat, dog, etc., or part of an animal like isolated heart, a strip of stomach, uterus, ileum, jejunum, colon, diaphragm, rectus abdominous muscle, etc., or blood cells or microorganisms. Unlike the physical and chemical methods, the bioassays are always comparative. The effect of the test substance is compared with that of the reference standard.
BIOASSAY OF INSULIN
- Standard Preparation and Unit: It is pure, dry, and crystalline insulin. One unit contains 0.04082 mg. This unit is specified by the Ministry of Health, Government of India, and is equivalent to an international unit.
- Preparation of Standard Solution: Accurately weigh 20 units of insulin and dissolve it in normal saline. Acidify it with HCl to pH 2.5. Add 0.5% phenol as preservative. Add 1.4% to 1.8% glycerin. The final volume should contain 20 units/ml. Store the solution in a cool place and use it within six months.
- Preparation of Test Sample Solution: The solution of the test sample is prepared in the same way as the standard solution.
Rabbit Method
- Selection of Rabbits: They should be healthy, weighing about 1800-3000 gm. They should then be maintained on a uniform diet but are fasted for 18 hrs. before assay. Water is withdrawn during the experiment.
- Standard and Sample Dilutions: These are freshly prepared by diluting with normal NaCl solution to contain 1 unit/ml. and 2 units/ml.
- Doses: The dose which can produce a suitable fall in blood sugar level is calculated for the standard.
- Principle: The potency of a test sample is estimated by comparing the hypoglycemic effect of the sample with that of the std. preparation of insulin.
- Experimental Procedure: Animals are divided into 4 groups of 3 rabbits each. The rabbits are then put into an animal holder. They should be handled with care to avoid excitement.
- The first part of the Test: A sample of blood is taken from the marginal ear vein of each rabbit. The presence of reducing sugar is estimated per 100 ml. of blood by a suitable chemical method. This concentration is called ‘Initial Blood Sugar Level’.
- The four groups of rabbits are then given sc. injections of insulin as follows:
- Any other suitable method can also be used.
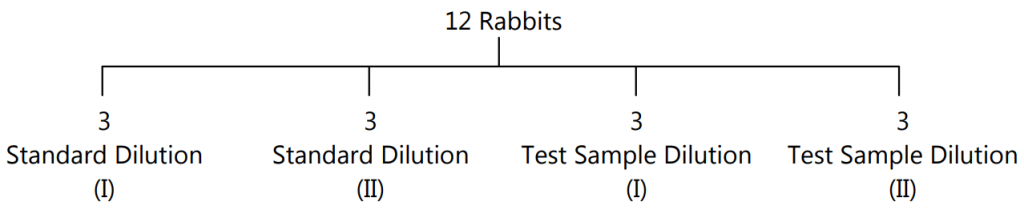
- From each rabbit, a sample of blood is withdrawn for up to 5 hrs. at the interval of 1 hr. each. Blood sugar is determined again. This is known as the ‘Final Blood Sugar Level’.
- The second part of the test (Cross over test): The same animals are used for the second part. The experiment can be carried out after one week.
- Again they fast and initial blood sugar is determined. The grouping is reversed, that is to say, those animals which received the standard are given the test and those which received the test are now given the standard. Those animals which received the less dose of the standard are given the higher dose of the test sample and vice-versa. This test is known as the ‘Twin Cross Over Test’.
Mouse Method
- Mice show characteristic convulsions after s.c. injection of insulin at elevated temperatures. The percentage of convulsions produced by the test and standard preparations are compared.
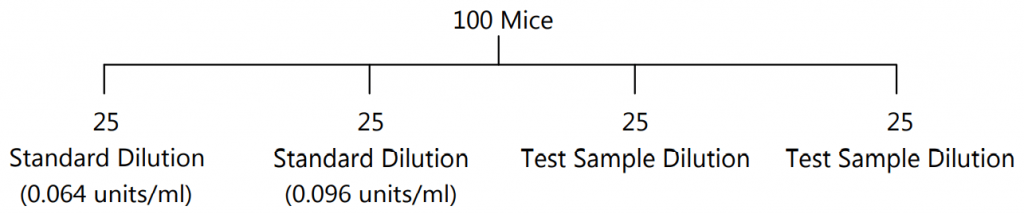
- Experimental Procedure: Minimum of 100 mice weighing between 18-22gm of the same strain are used. They should be maintained on a constant diet. They should fast 18 hrs. before the experiment.
- Standard and Sample Dilutions: Dilutions are prepared with sterile saline solution, to contain 0.064 units/ml. (std dilution I) and 0.096 units/ml. (std. dilution II). Similarly, test sample solutions are also prepared.
- Mice are divided into four groups each containing 25 mice and insulin is injected s.c. as follows:
- Mice are put in an air incubator at 33o C and observed for one and a half hr. The mice which convulse or die are taken out of the incubator and observed. These mice usually convulse severely but the failure of the animal to upright itself when placed on its back should as well be considered as convulsion.
Rat Diaphragm Method
- Sprague Dawley rats weighing 70–100 g are used. The animals are sacrificed during anesthesia and the diaphragms still attached to the rib cages are carefully removed, released from the rib cages, and adhering connective and fat tissues, washed in PBS, spread out, and divided into two equal pieces as described by Müller and coworkers (1994). For assaying the effects of insulin/compounds/drugs, the hemidiaphragm is incubated in KRH buffer gassed with carbogen (95% O2/5% CO2) in the presence of 5 mM glucose.
Epididymal fat pad of rats:
- Insulin-like activity can be measured by the uptake of glucose into fat cells. Adipose tissue from the epididymal fat pad of rats has been found to very suitable.
- The difference of glucose concentration in the medium after incubation of pieces of epididymal rat adipose tissue or measured oxygen consumption in Warburg vessels, Radiolabelled 14C glucose, the 14CO2 is trapped and counted.
- The concentration is determined by immuno-assay.
BIOASSAY OF OXYTOCIN
- Principle: The potency of oxytocin is determined by comparing its activity with that of the Standard Preparation of oxytocin under the conditions of a suitable method of assay.
- Standard Preparation: The Standard Preparation is the 4th International Standard for Oxytocin, established in 1978, consisting of freeze-dried synthetic oxytocin peptide with human albumin and citric acid (supplied in ampoules containing 12.5 Units), or another suitable preparation the potency of which has been determined to the International standard.
Method A:
- By depression of the blood pressure in chicken: Anaesthetise a young healthy adult cockerel weighing 1.2 to 2.3 kg with an anesthetic that will maintain an 18 prolonged and constant high blood pressure. Expose the gluteus primus muscle in one thigh and cut and retract it to reveal the popliteal artery and crural vein. Cannulate the popliteal artery and record the blood pressure on a suitable recorder calibrated for use over a linear range. Cannulate the crural or brachial vein. Immediately before use prepare a solution of the Standard Preparation in saline solution so that the volume to be injected is between 0.1 ml and 0.5 ml. Record the blood pressure responses to the injection into the cannulated vein of two doses of this solution; the doses should be such as to produce discriminated, precipitous, submaximal decreases in blood pressure; the required doses normally lie between 20 and 100 million Units. The interval between injections should be constant and lie between 3 and 10 minutes depending on the rate at which the blood pressure returns to normal. Immediately before use dilutes the preparation being examined with saline solution to obtain responses similar to those obtained with the Standard Preparation. The ratio between the two doses of the preparation being examined should be the same as that between the two doses of the Standard Preparation and this ratio should be kept constant throughout the assay. The two doses of the Standard Preparation and the two doses of the preparation being examined should be given according to a randomized block or a Latin square design and at least six responses to each should be recorded. If the animal rapidly becomes insensitive to the repeated injections of the solutions another animal must be used. Measure all the responses and calculate the result of the assay by standard statistical methods.
Method B:
- By contraction of the rat uterus: Inject 100 mg of oestradiol benzoate intramuscularly into a female rat weighing 120 to 200 g 18 to 24 hours before the assay. Immediately before the assay confirm by vaginal smear that the rat is in oestrus or proestrus. Kill the rat and suspend one horn of the uterus in a bath containing a solution of the following composition:
- Composition (% w/v)
- Sodium chloride 0.662
- Potassium chloride 0.045
- Calcium chloride 0.007
- Sodium bicarbonate 0.256
- Disodium hydrogen phosphate 0.029
- Sodium dihydrogen phosphate 0.003
- Magnesium chloride 0.010
- Dextrose 0.050
- Maintain the bath at a temperature of 32ᵒC or at some other suitable temperature at which spontaneous contractions of the uterus are abolished and the preparation maintains its sensitivity. Oxygenate the solution with a mixture of 95% of oxygen and 5% of carbon dioxide and record the contractions of the muscle using a suitable instrument giving a linear response (for example an isotonic lever with a load not exceeding 2 g). Record the contractions produced by the addition to the bath of two doses of the Standard Preparation suitably diluted with the above solution. The doses should be such as to produce discriminated, submaximal contractions; the required doses normally lie between 10 and 50 micro Units per ml of bath liquid. When maximal contraction has been reached, replace the bath liquid with a fresh solution. The doses should be added at regular intervals of 3 to 5 minutes depending upon the rate of recovery of the muscle.
- Dilute the preparation being examined to obtain responses on the addition of two doses similar to those obtained with the Standard Preparation. The ratio between the two doses of the preparation being examined should be the same as that between the two doses of the Standard Preparation and this ratio should be kept constant throughout the assay.
- The two doses of Standard Preparation and the two doses of the preparation being examined should be given according to a randomized block or a Latin square design and at least six responses to each should be recorded.
- Measure all the responses and calculate the result of the assay by standard statistical methods.
Method C:
- By measurement of milk-ejection pressure in a lactating rat: Select a lactating rat, in the third to the twenty-first day after parturition and weighing about 300 g, separate it from the litter and 30 to 60 minutes later anesthetize (for example, by the intraperitoneal injection of a solution of Pentobarbitone Sodium). Tie the rat to an operating table, maintained at 37̊C by its hind legs leaving the front legs free. Cannulate the trachea with a short polyethylene tube of internal diameter about 2.5 mm in such a manner to ensure a free airway; apply artificial respiration only if necessary. Cannulate an external jugular or femoral vein with a polyethylene tube of the internal diameter of about 0.4 mm which is filled with saline solution and closed with a pin. Shave the skin surrounding the inguinal and abdominal teats and excise the tip of one teat, preferably the lower inguinal teat. Insert a polyethylene tube of internal diameter about 0.3 mm and external diameter about 0.6 mm, to a depth sufficient to obtain an appropriate measurement of pressure (3 to 10 mm depth), into the primary teat duct which opens onto the cut surface and tie firmly in place with a ligature. Connect this cannula with a suitable strain gauge transducer (such as that used for recording arterial blood pressure in the rat) and fill the whole system with a 3.8% w/v solution of sodium citrate or saline solution containing 50 Units of heparin sodium per ml to prevent clotting of milk. After cannulation, inject a small volume (0.05 to 0.2 ml) of this solution into the teat duct through the transducer to clear the milk from the tip of the cannula. (This procedure may be repeated during the assay should obstruction arise from milk ejected into the cannula). Clamp the strain gauge so that slight tension is applied to the teat and its natural alignment is preserved and connect the gauge to a potentiometric recorder adjusted to give full-scale deflection for an increase in milk-ejection pressure of about 5.3 kPa. Inject all solutions through the venous cannula using a 1-ml syringe graduated in 0.01 ml and wash them in with 0.2 ml of saline solution. Prepare a solution of the Standard Preparation and a solution of the preparation being examined in saline solution so that the volume to be injected is between 0.1 ml and 0.4 ml. Choose two doses of the Standard Preparation such that the increase in milk-ejection pressure is about 1.35 kPa for the lower dose and about 2.7 kPa for the higher dose. As an initial proximation, a lower dose of between 0.1 and 0.4 milliUnit and an upper dose of 1.5 to 2 times this amount may be tried. Choose two doses of the preparation being examined with the same inter-dose ratio, matching the effects of the doses of the Standard Preparation as closely as possible. Inject the four doses (two doses of the Standard Preparation and two doses of the preparation being examined) at intervals of 3 to 5 minutes. The two doses of Standard Preparation and the two doses of the preparation being examined should be given according to a randomized lock or a Latin square design and at least four responses to each should be recorded. Measure all the responses and calculate the result of the assay by standard statistical methods.
BIOASSAY OF DIGITALIS
- Principle: The potency of the test sample is compared with that of the standard preparation by determining the action on the cardiac muscle. Any other equivalent method, which gives results similar to those obtained by this method is also valid.
- Standard Preparation and Units: The standard preparation is a mixture of dried and powdered digitalis leaves (1 unit = 76 mg.)
- Preparation of Extracts: Exact amount of the powder is extracted with dehydrated alcohol in a continuous extraction apparatus for six hours. The final extract should contain 10 ml. (5 ml. alcohol + 5 ml. water) per 10 g. of digitalis powder. It should be stored between 5 ̊ C and –5 ̊C.Bioassays
1. Guinea–pig Method (Endpoint method):
- Standard and test sample extracts are diluted with normal saline in such a way that 1 g of digitalis powder is diluted to 80 ml. A guinea pig is anesthetized with a suitable anesthetic. It is dissected on the operation table.
- The jugular vein is traced out by removing adhering tissues and cannulated using a venous cannula. A pin is inserted in the heart, such that it gets inserted in the apex of the heart. In this way, we can observe the heartbeats by the up and down movements of the pin.
- The injection is continued through the venous cannula until the heart is arrested in systole. The amount of extract required to produce this effect is taken as the lethal dose of the extract.
- Another set of 19 animals of the same species are used for this experiment and the average lethal dose is determined.
- It is not necessary to determine the lethal dose of the std. during each time of the experiment. But it should be occasionally checked.
- The lethal dose of the test sample is determined in a similar way using a minimum of 6 guinea–pigs of the same strain.
- The potency of the test sample is calculated to that of the std. preparation by dividing the average lethal dose of the sample to the test and expressed as units per gram.
2. Pigeon Method:
- Minimum 6 pigeons are used for testing each sample. They should be free from gross evidence of disease or emaciation.
- The weight of the heaviest pigeon should not exceed twice the weight of the lightest pigeon. Food is withheld 16-28 hours before the experiment. Pigeons are divided based on their sex, weight, and breed, into two groups.
- They are anesthetized with anesthetic ether. One side of the wing is dissected and the alar vein is cannulated using a venous cannula. Dilutions are made with normal saline. An average lethal dose of each sample is determined; results are tabulated and calculated as per the guinea pig method.
- The lethal dose per kg. of body weight is determined for each pigeon. The potency of the test sample is determined by dividing the mean lethal dose of the standard by the mean lethal dose of the test sample.
- In pigeons, the stoppage of the heart is associated with a characteristic vomiting response called ‘emesis’. The milk from the crop sac of pigeons is being ejected out. This may be taken as the endpoint response of digitalis.

BIOASSAY OF D-TUBOCURARINE
Rabbit Head-drop Method
- Principle: d-Tubocararine hydrochloride is injected into the marginal vein of a rabbit’s ear till the rabbit’s neck muscles are relaxed such that the animal cannot hold its head up. The total amount of test sample required to produce the endpoint is compared with the total amount of the standard sample required to produce a similar endpoint.
- Selection of Rabbits: Rabbits weighing 2 kg. are used. Animals should be free from disease, obtained from a healthy colony, and should be accustomed to the experimental procedure.
- Experimental Procedure: Each rabbit is placed in a holder with its head protruding outside. The head should be freely movable. Minimum 8 rabbits are used. They are divided into two groups each containing 4 rabbits. The first group will receive a standard sample and the second group will receive the sample under test. the d-Tubocurarine solution is injected at a constant speed by infusion apparatus through the marginal vein.
- i.v. inj. of d-tubocurarine.
- Head drops after injection. Injection should be given at a rate of 0.4 ml/min and should take about 10 min. Infusion is continued till the rabbit will not be in a position to hold its head erect or there will be no response by focusing light on the eyes and the neck gets elongated and toneless.
- Suitable dose of d-tubocurarine is 0.012% w/v in saline. Rabbits recover immediately from the effect of curarization. During the expt. there is a possibility of respiratory embarrassment which is treated by injecting neostigmine methyl sulfate (0.05 mg.) and atropine sulfate immediately through the marginal ear vein.
- A cross-over test is carried out to minimize biological error due to animal variation. Those rabbits which received the standard sample on the first day will be given a test sample on the second day of expt. and vice versa. The mean dose which produces the head drop of the test sample is compared with the mean dose of the standard preparation.
Frog’s Rectus Abdominis muscle Preparation
- A frog is pithed and laid on its back on a cork-covered board to which it is pinned. The skin covering the abdomen is cut away and the rectus abdominal muscle of one side is dissected from the pelvic girdle to its insertion in the cartilage of the pectoral girdle. The muscle is then pinned to the cork by four pins to keep its normal length while a thread is sewn through each end. It is then mounted in the organ bath containing frog’s Ringer solution which contains:
- NaCl, 6.5gm.; KCl, 0.29 gm.; CaCl2, 0.24 gm.; NaHCO3, 0.4 gm.; glucose, 1.5 gm. and distilled water 2000 ml.
- Oxygenation is carried out to keep the tissue alive. The muscle is stabilized for 30-45 min. to get a critical quantitative response. The responses are recorded using an isotonic frontal writing lever with 1 G. tension.
- Two similar contractions with the same concentration of acetylcholine are obtained. Three doses of the standard sample and one intermediate dose of the test sample are selected and the reduction in height of contraction induced by acetylcholine is noted down.
- Acetylcholine contraction is recorded on the slow-moving drum for 90 sec. d-Tubocurarine is allowed to act for 30 sec. The percentage reduction at each dose level is calculated and the log dose-response curve of the standard drug is plotted. A linear response will be obtained. The potency of the test sample is calculated from the standard curve.
BIOASSAY OF HISTAMINE
Bioassay of Histamine using guinea-pig Ileum:
Drugs:
- Standard histamine solution (St.) (10 µg/ml)
- Test histamine solution (T)
Procedure:
- A clean strip of the ileum is placed in freshly prepared Tyrode’s solution. A small segment (3cm) of ileum is cut; a thread is passed through the lumen & the wall at each end of the segment with the aid of a fine needle.
- One end of the segment is tied securely to the aeration tube & transferred to the organ bath (already filled with Tyrode’s solution & bubbled with gas). The other end of the ileum is attached to the transducer; the tension of the thread is adjusted.
- The baseline record of contraction on the physiograph and the sensitivity are adjusted before the addition of drugs.
- The normal tone of the ileum is recorded for 1 min. then different volumes of St. histamine are started to be added. The contact time for each histamine volume is 30 seconds. At the end of each cycle, the tissue is washed two times with Tyrode’s solution each time the tissue is allowed in contact with the solution for 30 seconds.(Cycle time= 2.5min, contact time for agonist(30 sec.) + normal record(1min.)+ washing twice(30 sec.))
- Different doses of St. histamine solution are added (0.05ml, 0.1ml, 0.2ml, 0.3ml &0.4ml) to chose two proper doses and bracketing the test in between them. The amount of standard which produces responses matching those of the dose of the test is tried and an attempt is made to reduce the limits as far as possible.
- Changing in St. histamine doses, followed by the dose of test, is continued till the test dose was bracketed between 2 doses of St. histamine given nearly the same response of the test.
BIOASSAY OF VASOPRESSIN
Definition:
- Bioassay is defined as the estimation of the concentration or potency of a substance by measuring its biological response in a living system.
Principle:
- The potency of vasopressin injection is determined by comparing test activity with that of a standard preparation of vasopressin.
Standard Preparation:
- It is a dried acetone extract of posterior lobes of the pituitary gland of oxen or any other suitable preparation.
- Standard unit: Specific pressor activity corresponding to that yielded by 0.0005gm of standard preparation (20units/ml).
Procedure:
- Animal: Albino rat of 300g weight.
- Anesthetize it by S.C. injection of Ethyl carbamate.
- After 40-60 min., cannulate the trachea with a polyethylene tube of 2.5 mm external diameter.
- Dissect carotid artery for cannulation.
- Cannulate femoral vein close to inguinal ligament by the following process:
- Retract abdominal muscles to expose the inguinal ligament and superficial prudential vein to one side.
- Dissect femoral vein towards inguinal ligament from the corresponding artery.
- Tie a short polyethylene cannula (1 mm external diameter) into a femoral vein by two ligatures, joined by a short piece of rubber tubing to 1 ml burette with an attached thistle funnel containing saline solution.
- Fix a wet cotton swab & tie to cover the incision and cannula.
- Inject 200U heparin in saline solution/100 g body weight.
- Connect carotid artery cannula with mercury manometer (2-3mm internal diameter).
- Inject all solutions through the venous cannula with a 1 ml syringe.
- A suitable hypotensive agent is given into the tail vein to produce a constant basal pressure of 50 torrs.
- Dilute standard & test preparations such that the volume to be injected is between 0.1- 0.5 ml.
- Choose 2 doses of standard so that lower dose produces 30 torr B.P. & higher produces 50 torr B.P.
- i.e., the ratio of doses should be 3:5.
- Select test doses according to standard doses.
- Doses are added at intervals of 3-5 min. in random order.
- Record rise in B.P. in response to each dose.
Method 2:
- Anesthetize a healthy cat with a volatile anesthetic agent.
- Insert a tracheal tube for artificial respiration.
- Expose spinal cord from behind by removing second cervical vertebrae.
- Destroy brain bypassing suitable instrument through the foramen magnum.
- Start artificial respiration through tracheal tube & leave animal for an hour to remove the anesthetic effect.
- Cannulate carotid artery for B.P. measurement & femoral vein for injection of drug solutions.
- Maintain normal B.P. at 50-100 torr.
- Select 2 doses of test & standard, inject 0.05-0.1 units at 30 min. interval.
- Record maximum rise in B.P. in response to each dose.
BIOASSAY OF ACTH
Official preparation
- Corticotropin injection: is a sterile solution, in suitable diluents, of the polypeptide from the pituitary glands of mammals. The potency range should be 80.0 – 120.0% of corticotropin units.
Purpose and Rationale:
- This is a historical assay method.
- Administration of pituitary gland ACTH decreases the ascorbic acid present in the adrenals.
- The depletion of adrenal ascorbic acid is a function of the dose of ACTH administered.
- This relationship has been used for a quantitative assay of ACTH.
Solution:
- Five units of test or standard dissolved in 0.25 ml of 0.5% phenol solution and diluted with 8.1 ml of 15% gelatin solution (Now 0.5 ml contains 300 mU ACTH). (Solution A)
- Three ml of solution A diluted with 6 ml of gelatin solution. Now concentration reduced to 100 mU ACTH/0.5 ml) (Solution B).
- Again 3 ml of solution B diluted with 6 ml of gelatin solution, the resulting solution contains 33 mUACTH/0.5 ml.
Procedure:
- Male Wistar rats (100-200 g) are hypophysectomized (pituitary gland removed by surgery) one day before the test.
- For one test with 3 doses of test preparation and standard.
- Several hypophysectomized rats are required: at least 36 (preferably 60).
- The hypophysectomized rats are randomly distributed into six groups. Each rat receives subcutaneous 0.5 ml of the various concentration of test or standard.
- Three hours after injection, the animals are anesthetized and both adrenal and removed, freed from extraneous tissue, and weighed. The rats are sacrificed and the skull opened to verify completeness of hypophysectomy.
- The adrenal is homogenized in glass tubes contains 200 mg pure sand and 8.0 ml of 4% trichloroacetic acid and the ascorbic acid determined.
- The potency ratio including confidence limits is calculated with the 3+3 point assay.
Estimation of Ascorbic Acid:
- Preparation of 1 mg/ml concentrated. Of ascorbic acid in 4% TCA (stock) solution A.
- Use solution A to prepare 0.2% of ascorbic acid in 4% TCA (solution B).
- Use solution B to prepare 0.02% of ascorbic acid in 4% TCA (solution B).
- The calibration curve is established at a wavelength of 540 mm using the solutions without ascorbic acid as blank.
BIOASSAY OF 5HT
Objective:
To record the concentration-response curve of 5 HT using isolated rat fundus strip preparation.
Principle:
- The basic principle of bioassay is to compare the test substance with the Standard preparation of the same and to find out how much test substance is required to produce the same biological effect, as produced by the standard.
- Rat fundus is a very sensitive tissue for the study of the action of several naturally occurring substances like 5HT, Histamine, Acetyl Choline, and Bradykinin.
- Unlike the intestinal smooth muscle, this preparation is slow contracting and slow relaxing serotonin.
- Rat fundus preparation is generally employed for the bioassay of serotonin.
- The fundus is grey and therefore, easily identified from the pyloric part.
- A zig-zag preparation of the fundal strip is prepared to expose the maximum portion of the tissue to the drug.
- The tissue is sensitive to 1 ng/ml of serotonin.
Procedure:
1. Sacrifice the rat by a blow on the head and carotid bleeding.
2. Cut open the abdomen and expose the stomach.
3. Identify the fundus of the stomach, incise it from the junction of the pyloric part, and put it in the dish containing Krebs solution.
4. Incise the fundus from the lesser curvature and open it longitudinally. Give alternate Zig-Zag cuts to make a fundal strip preparation, tie both the ends with thread, and mount in the organ bath containing Krebs solution at 37˚C. Aerate the tissue.
5. Apply 1g load and allow the preparation to equilibrate for 30 min. using frontal writing lever with 10-12 magnification record the contraction due to increasing concentration of serotonin. Since the muscle contracts slowly and relaxes slowly, a contact time of 90sec and a 5 min time cycle are followed for proper recording of the concentration-response curve.
6. Label and fix the tracing. Plot the concentration-response curve.
Make sure you also check our other amazing Article on : Drugs Acting on the Uterus