Chromatography is the separation technique used for the separation of a complex mixture. For example, in a tablet, the active ingredient is mixed with various excipients. In this, chromatography will be helpful to separate the excipient with other ingredients. Similarly, in-plant extract there are various phytoconstituents are present. The chromatography will be helpful to separate these phytoconstituents into their individuals.
Chromatography word was derived from the two words chroma and graphy. Chroma the greek word indicates colour and graphy represents the meaning of writing and recording. The person who invented chromatography first was a Russian botanist Mikhail Tswett. He separated the chlorophyll and xanthophylls from the leaves of the plant by using a glass column packed with the fine particle of calcium carbonate. So the chromatography is a very primitive technique used for the separation of the mixtures.
Column Chromatography
Table of Contents
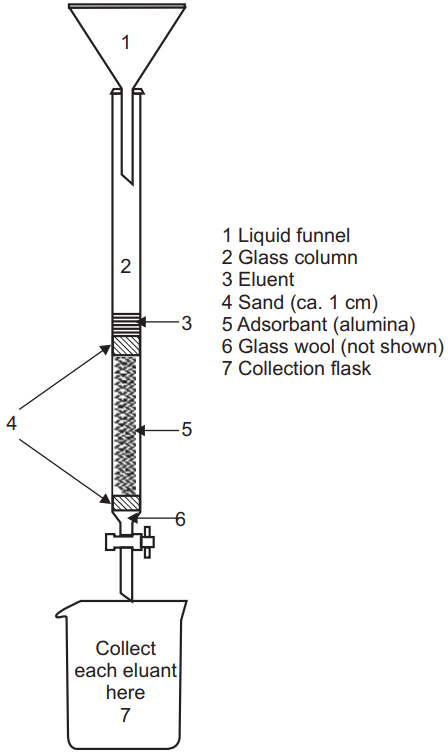
Generally in all the types of chromatography, there is one stationary phase and one mobile phase. In column chromatography, we use a packed column as a stationary phase. The column may be packed with solid or liquid. If the packing occurs with the solid the principle of separation is adsorption while if packing will be done by liquid then the principle of separation will be partition. Generally in column chromatography, the most separation is based on the adsorption principle. The adsorption principle is used in the separation of the mixture from column chromatography. The extract which is to be analyzed should be dissolved in the mobile phase and added into the column. The individual constituent in the extract will move with different rates which depend upon their affinities towards the stationary phase or adsorbent. The compounds which have more affinity towards the stationary phase will be separated in the last while those that have less affinity towards the stationary phase will move fast and separated first.
The stationary phase which is used to fill the column should have a uniform spherical size with high mechanical stability, inert with solute or other components, and the mobile phase has a free flow, freely available, inexpensive, and able to separate the wide variety of compounds.
Example of weak adsorbent is sucrose, starch, inuline, talc while medium adsorbent is calcium carbonate, magnesium oxide, calcium hydroxide, and magnesium carbonate and the example of strong adsorbent is activated alumina, activated charcoal, fuller’s earth, etc.
The mobile phase may be used in individuals or can be used in the mixture depending upon their polarity and the type of constituent to be separated. The column should be made of neutral glass and not affected by acid, alkali, mobile phase, or stationary phase. The length: diameter ratio may be 10:1 to 30:1. Sometimes it may be a 100:1 ratio.
Packing of the Column:
The lower portion of the column should be packed with glass wool or cotton wool above which the adsorbent should be placed. After packing with the adsorbent, a paper disc can be placed above the adsorbent layer. So that the introduction of the sample or mobile phase will not disturb the adsorbent layer. There are generally two types of column packing techniques
- Dry packing technique.
- Wet packing technique.
In the dry packing technique, the column is packed with dry adsorbent and then flown the solvent through the column till equilibrium is obtained but the major drawback is that air bubbles are trapped between the solvent and adsorbent which produces trouble in the separation of compounds.
In the wet packing technique, the mobile phase is mixed with adsorbent and poured into the column. So the air entrapment is comparatively less and uniform packing occurs in the column. If the flow of the mobile phase will be uniform there will be no development of crack and good and uniform separation of the extract will occur.
Sample Introduction:
The sample should be dissolved in the least quantity of mobile phase. The complete sample should be placed on the top portion of the column and get them absorbed. Then the sample can be isolated by elution. Elution may be of two types:
- In Isocratic elution, the composition of the mobile phase will be the same for the entire separation.
- In gradient elution, the composition of the mobile phase can be changed slowly to improve the separation. The polarity of the mobile phase can be changed by changing the mobile phase combination.
Detection of Components:
Colored constituents can be detected visually and colored bands moving in the column can be collected separately. A colourless compound can be detected by UV technique, refractive index detector, monitoring by TLC, by flame ionization detector, or such other technique. The component can be recovered by different techniques some time even by cutting the column (column made of plastic) into several distinct zones but the best method is elution in which the component is eluted by the mobile phase and the constituent is separated from the mobile phase.
Paper Chromatography
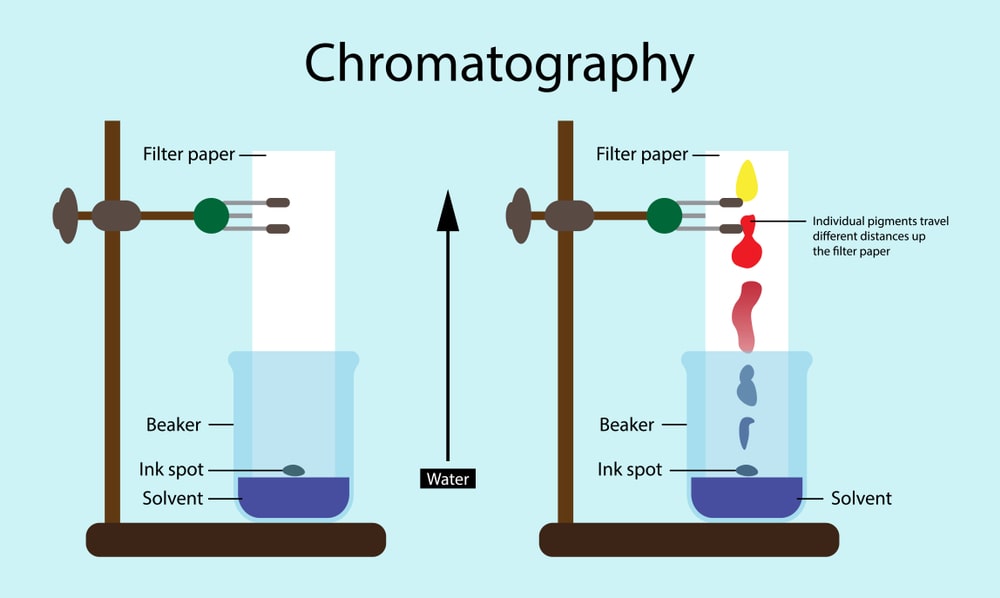
Paper chromatography is carried out by specially designed filter paper. The principle of separation may be here partition or adsorption. If the filter paper is impregnated with alumina or silica, the adsorption principle will be applied for separation whereas if moisture/water is present in the pores of cellulose fiber it works as stationary phase and solvent as mobile phase then the principle of separation will be partition. In the general paper, chromatography refers to the partition principle.
Various types of filter paper are used for paper chromatography. It may be Whatmann paper of different grade, acid or base wash filter paper, paper modified with glycol, formamide, methanol, glass fiber type paper, hydrophobic paper (OH group can be acetylated) or paper can be impregnated with alumina, silica, or ion exchange resin.
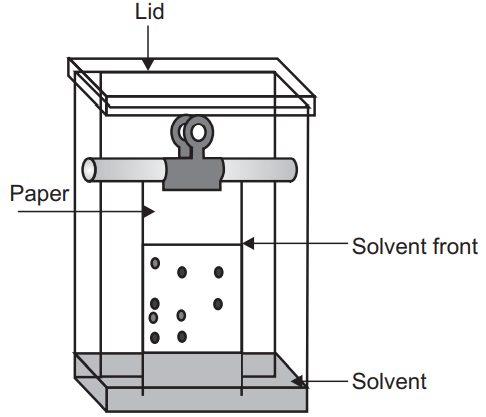
The size of the paper should be suitable for the size of the chamber and apply the sample by using a capillary or micropipette. The sample should be dissolved in the mobile phase and applied with low concentration in the small zone. In the mobile phase, pure solvent or a mixture of solvent or buffer solution can be used.
There may be ascending, descending, circular, two dimensional, and ascending descending types of different development techniques.
Ascending development technique is a conventional technique in which the mobile phase moves against gravity and spot the sample at the bottom portion. While in descending development the mobile phase is kept at the top and the solvent flows down the paper. Here the samples are applied at the top and the development is fast due to gravity-assisted solvent flow.
In circular or radiant development the sample is applied at the center of the paper and the mobile phase is flown through a wick at the center and spread uniformly in all directions. In two-dimensional development techniques, the samples are applied in one corner and develop the paper on one axis then dry the paper. After drying turn the paper on a ninety-degree angle and develop the paper on another axis. This technique is used for more complex samples. By ascending descending development the length of separation can be increased. First here ascending takes place later descending development follows.
After the development of the chromatogram, the isolated compound can be visualized by detecting agents. Detecting agents can be two types:
- Destructive type
- Non-destructive type
In destructive type, the sample cannot be recovered or it will be destroyed due to the chemical reaction of spraying reagent with the sample. While in the non-destructive method sample can be recovered. In the non-destructive method, a sample can be detected by the UV chamber method, densitometric method, or iodine chamber method.
Paper chromatography can be used for both qualitative and quantitative purposes. For qualitative purposes, the Rf value can be determined.

For the quantitative purpose, the density of the spot can be measured or the spot can be eluted with the solvent and analyzed by conventional techniques like spectrophotometric method or electrochemical methods.
Thin Layer Chromatography (TLC)
Stahl in 1958 developed the standard equipment and technique for analysis by thin-layer chromatography. Before that in 1938 Izmailou and Shraiber separate the phytoconstituent using 2 mm thick alumina on a glass plate. TLC is a comparatively better separation tool than paper chromatography. Though the adsorbent term use various times in the TLC principle of separation may be any one of four fundamental mechanisms of separation:
- Ion exchange
- Partition
- Adsorption
- Molecular exclusion
The principle of separation depends upon the coating substance applied on the plate. If silica or alumina is applied as a coating substance adsorption principle will take place. If the coating substance is Sephadex molecular exclusion will be a principle of separation. Ion exchange will be the mechanism of separation if resin will be the coating substance and cellulose-like coating substance adopt partition mechanism.
The extract of the plant is spotted on the plate and it moves along with the mobile phase. The phytoconstituent of the extract has a different affinity towards the stationary phase and mobile phase. Those constituents have more affinity towards mobile phase separation faster compared to those who have more affinity with coating substances.
Silica gel G is one of the most common adsorbents used for the coating of plates. Here G represents the gypsum (CaSO4) which is around 15 percent of the silica gel and acts as a binder. The other adsorbent which is used in the stationary phase is alumina, cellulose, kiesselguhr, polyamide powder, and others. Most often the adsorbent is applied to the glass plate. The dimension of the glass plate may vary from 20X20 cm to 20X5 cm. sometimes even microscopic plates are also used to examine the progress of the chemical reaction by the TLC method.
The glasses are coated with adsorbents with different techniques like 1. Pouring, 2. Dipping, 3. Spraying, 4. Spreading techniques.
In the pouring technique, the prepared slurry is poured onto the glass plate and tries to maintain an equal level surface. In the dipping technique, two plates are stick together and dip into the slurry and then remove from the slurry. Separate both plates, one surface will be coated and the other one will be dried. In the spray technique adsorbent is sprayed on a glass plate using a mechanical sprayer. The best applying technique is the spreading technique in which the slurry is kept in the TLC spreader box. The glass plates of a particular size are kept on the base plate. The prepared slurry is kept in the reservoir of the spreader box. Adjust the thickness of the slurry by rotating the knob present in the spreader box then the spreader box is moved over the glass plate. Allow them for air drying and activate them at 100-120°C for one hour.
The sample can be applied by micropipette or capillary tube. It should be spotted at least 2 cm above the base of or at such height that the spotted area should not be immersed in the mobile phase. The area of the spotted sample should be minimum with sufficient concentration. The mobile phase and TLC plate should be kept in the development chamber. The chamber should be saturated with mobile phase otherwise edge effect may be found in which the solvent front in the middle of the plate moves faster than the edge. The mobile phase selected is based on the phytoconstituent which has to be separated and the nature of the stationary phase. It may be a single or a mixture of the solvent. The polarity of the solvent was adjusted such that it easily separated the phytoconstituents.
The different development techniques used in the TLC are one-dimensional, two-dimensional, horizontal, and multiple developmental techniques. In one dimensional or vertical technique, the mobile phase flows against the gravity due to the capillary action. Most separations are done by this technique in which one spot is applied at the corner of the plate and the plates are developed in one axis then dried. Further, turn the plate to 90 degrees and develop the plate on another axis. After the development of the chromatogram, the isolated compound can be visualized by detecting agents. Detecting agents can be two types a) Destructive type b) Non-destructive type.
In the destructive type, the sample cannot be recovered or it will be destroyed due to the chemical reaction of spraying reagent with a sample. While in the non-destructive method sample can be recovered. In the non-destructive method, samples can be detected by the UV chamber method, densitometric method, or iodine chamber method.
This chromatography can be used for both qualitative and quantitative purposes. For qualitative purposes, the Rf value can be determined.

For the quantitative purpose, the density of the spot can be measured or the spot can be eluted with the solvent and analyzed by conventional techniques like spectrophotometric method or electrochemical methods.
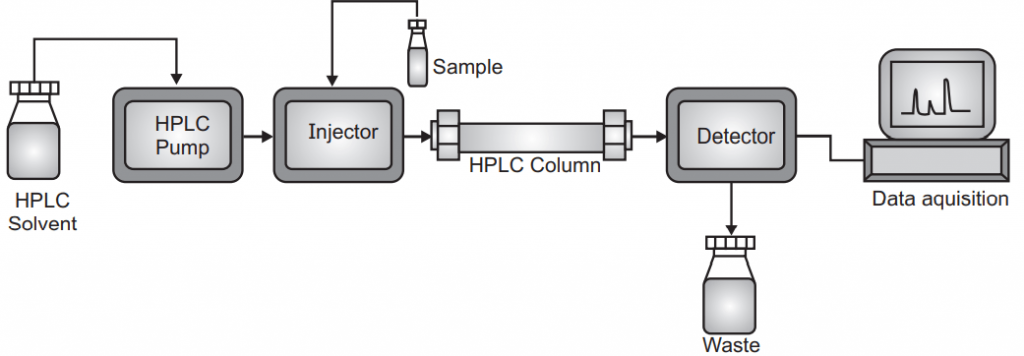
High-Pressure Liquid Chromatography (HPLC)
Compare to classical chromatography high-pressure liquid chromatography or high-performance liquid chromatography has much better performance. Compare to classical column chromatography it works under high pressure. HPLC has a smaller particle size for the adsorbent phase so that it provides more surface area which improves the separation.
In HPLC the particle size of stationary phase is less (3-20µm) with a small metal column packed under high pressure which run under high plow rate and high pressure (>5000 psi) while in conventional column chromatography particle size is less (60-200µm) with glass column packed under gravity pressure, run at very low flow rate and work under low pressure (< 20psi).
HPLC runs under normal phase mode or reverse-phase mode which depends upon the polarity of stationary phase and mobile phase. In normal phase mode, the mobile phase is non-polar and the stationary phase is polar while in the reverse phase mode mobile phase is polar and the stationary phase is non-polar.
The separation technique in HPLC may be isocratic or gradient. In the isocratic separation technique, the polarity of the mobile phase is maintained the same throughout the process while in gradient separation the polarity of the mobile phase can be gradually changed by changing the ratio of the different solvents present in the mobile phase.
The HPLC unit consists of different parts like a pump, mixing unit, injector, guard column, analytical column, detectors, and recorder. The solvent is pushed into the column under very high pressure (1000 to 5000 psi) because the particle size of the stationary phase is very small (5-10 µm) so the resistance force by mobile phase is very high. To generate such pressure mechanical or pneumatic pumps are used. The mechanical pump works under constant flow rate and for generally analytical purposes. Pneumatic pumps work under constant pressure with highly compressed air. Flow rate and back pressure are controlled by the check valve. The pulse generated by the pump is dampened by pulse dampeners. In the mobile phase, there may be a single solvent or maybe more than one solvent. To mix properly more than one solvent there is a mixing unit in the HPLC apparatus. It may be two types a) low pressure mixing chamber b) high pressure mixing chamber. In a low-pressure mixing chamber, helium is used for degassing the solvent. The quality of solvent should be very good or maybe HPLC grade.
Various gases are soluble in the mobile phase. Mobile phase under high pressure, when pumped in soluble gas, becomes undissolved and forms the bubble which interferes with the separation of constituents. To remove this hindrance the degassing of the mobile phase is very important. For degassing the various techniques like vacuum filtration, helium purging, ultrasonication can be used. Vacuum filtration is not very much reliable, helium purging is efficient but helium is costly gas so that ultrasonication is the better technique. Ultrasonicator removes the air bubble into the mobile phase by converting ultra-high frequency into mechanical vibration.
The sample can be introduced into the HPLC by manual method or auto-injection method. It can be septum injection, stop flow (on line) injection, or loop valve (Rheodyne injectors). In septum injection, one rubber septum is used to inject the sample but the septum should withstand high pressure, and leaching (erosion of rubber) also exist. Sometimes stop the flow of the mobile phase and introduce the sample through a valve device but the most popular is the loop valve (Rheodyne) injector type. This first sample is loaded on the injector (20- 50 µl) which is the load position and then inject the sample without disturbing the flow of mobile through inject mode.
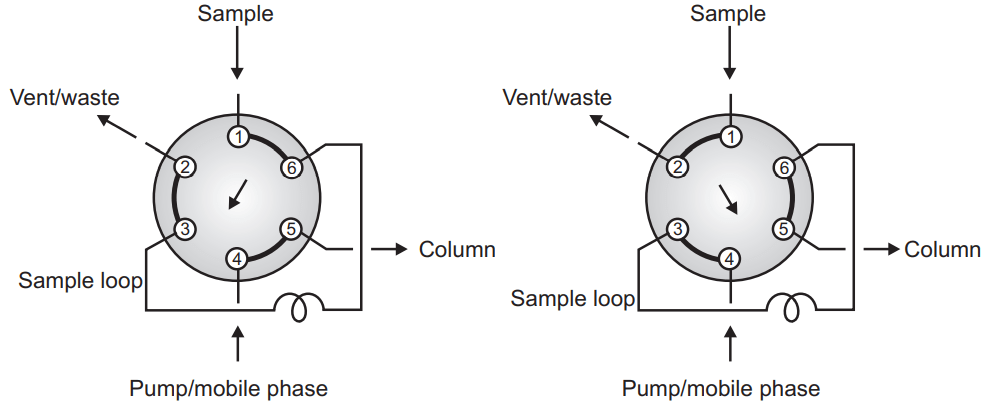
A very important part of the HPLC is the analytical column but before the analytical column, one guard column is also provided which is comparatively cheaper than the analytical column and helpful to increase the life of the analytical column by removing the entry of unwanted material in the analytical column. The performance of the analytical column decides the efficiency of separation. The column can be made of polyether ketone, stainless steel, polyethylene, or sometimes glass. Stainless steel can be the most widely used column because it can withstand high pressure compared to the PEER column. The column length can vary from 5-30 cm with a diameter of 2-50 mm using an adsorbent particle size of 1 µm to 20 µm with a uniform spherical porous material. 1gm stationary phase may have an average 400 sqm area.
The detector used in the HPLC can be divided into two categories, the solute property detector, and the bulk property detector. A bulk property detector also known as a universal detector, measure the characteristic of all analyte by analyzing mobile phase without or with a sample. Good examples of these are conductivity detectors or refractive index detectors. Solute property detector corresponds to the particular unique property of the analyte like UV detector, Fluorimetric detector, Photodiode array detector.
The response of the separated constituents can be recorded by the recorder. Recorder amplifies the response which is detected by the detector. It records the time at which the constituent is separated or retention time. The integrator measures the height and width of the peaks, peak area, and percentage of the area.
HPLC can be used for the qualitative analysis by measuring the retention time of the sample under standard conditions but generally, it is used for quantitative analysis by direct comparison method, calibration curve method, and by internal standard method.
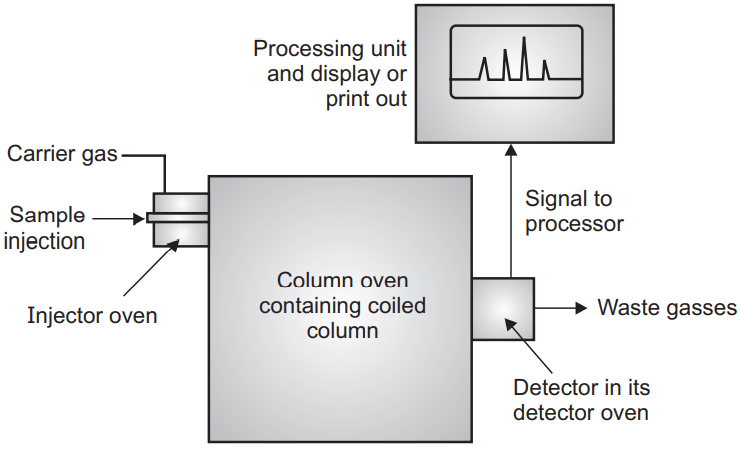
Gas-Liquid Chromatography (GLC)
Gas chromatography is generally divided into GSC (gas-solid chromatography) and GLC (gas-liquid chromatography). In either type, the gas is a mobile phase but the stationary phase varies in GC (gas chromatography). In GLC stationary phase is liquid and in GSC it is solid. In GLC the principle of separation is partition while in GSC it is adsorption. Most of the time when named GC it is GLC.
Generally, a liquid is coated on solid support used as a stationary phase. The mixture which has to separate into the individual constituent has to be converted into the vapor and mixed with the mobile phase (gas). The constituent which has more affinity towards the stationary phase travels slowly compare to those which have a high affinity towards the mobile phase. The constituents are separated based on their partition coefficient. GLC is a good technique to detect those compounds which are volatile and thermostable.
The volatile compound should be mixed with the carrier gas. The carrier gas may be hydrogen, helium, nitrogen. A hydrogen is a good option for carrier gas because of its good thermal conductivity and low density but it reacts with unsaturated compounds. Helium is also a good choice but it is expensive. Nitrogen is inexpensive but has reduced sensitivity. Gases are generally stored under high pressure. To blow the gases under uniform pressure and flow rate there is a need for a flow meter. Generally, rotameter and soap bubble meters are used to control the flow of gases.
The sample can be introduced into any form like solid, liquid, or gaseous form. Valves are suitable devices to introduce the gas sample. Solid samples are generally dissolved in an appropriate solvent and then injected through a septum. Liquid samples can be dispensed through either loop or septum devices. Septum should be made of high-quality silicone rubber and can tolerate high temperature and is suitable for repeated injection.
Another important part of GLC which effect the separation of the constituent is a column. They are made of either glass or stainless steel. The stainless steel column has a long life and is easy to handle but sometimes it may react to the constituent which is not in the case of glass columns but glass columns are fragile and difficult to handle. The column may be analytical (length 1-1.5 mt, diameter 3-6 mm) or preparative column (length 3-6 mt, outer diameter 6-9 mm). Depending upon its nature it may be a packed column, open tubular or Golay or capillary column and support coated open tubular column (SCOT).
Though the sample should be converted into the vapor the pre-heaters are required in the GLC which converts the sample into vapor form and mix with the carrier gas. They are installed along with injecting device. A thermostatically controlled oven can be used for this purpose which can operate on an isothermal programming base (same temperature during entire operation) and linear programming (oven is heated linearly over some time).
Different type of detector like kathrometer, FID (flame ionization detector), AID (argon ionization detector), ECD (electron captured detector) are used. The most sensitive of them are ECD (10-12). Recorders record the response and amplify it. Recorder record the retention time, record baseline, and record all the peaks. Heights, width, area of the individual peak, percentage of area are calculated by integrators.
The separation or detection of the sample can be improved in GLC by derivatization techniques. It can be precolumn derivatization or post-column derivatization. In precolumn derivatization, the sample is converted into more volatile and thermostable derivatives (like carboxylic acid, phenols, sugars are converted into less polar by reagent BSA bis trimethyl silyl acetamide). Post-column derivatization is done generally to improve the detector response for isolated constituents.
GLC can be used for the qualitative analysis by measuring the retention time of the sample under standard conditions but generally, it is used for quantitative analysis by direct comparison method, calibration curve method, and by internal standard method.
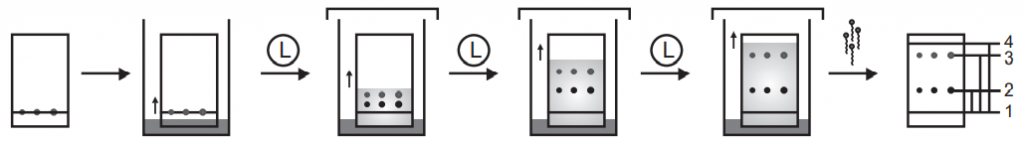
High-Performance Thin Layer Chromatography (HPTLC)
In contrast to the column chromatography (like GLC, HPLC) planar chromatography utilizes a planar (flat) stationary phase for the separation.
Sample Preparation:
The sample should be homogenous and representative of the entire batch. The sample should be prepared such as that analyte present in the traces quantity can be detected. If the sample is pure and concentrated can be directly applied to the HPTLC plate but if the analyte is present at less concentration with impurities than purification, isolation of analyte from interfering reagent and concentration procedure should be taken.
Sample Application:
The physical dimension of the isolated sample should be very compact. The optimum amount of the sample should be applied to the stationary phase. In the case of manual application, care should be taken that manual application does not damage the surface of the layer. During sample application, chemical fumes and vapors should be absent and temperature and humidity should be constant. A fully automatic sample application is better. It dispenses precise volume on precise position with precise delivery rate. Generally, 0.5-5µl sample applied in case of spot or 2-10µl as narrow band. Sample can be applied as 1) Manual application 2) Instrumental techniques 3) Semiautomatic application. The adverse effect of overloading of samples shows unresolved components.
Stationary phase, TLC plates, and solvents
Silica gel is the most popular solvent used in HPTLC afterward cellulose. Homemade sheets/plates show bigger particle and are not shows homogenous so it is not suitable for HPTLC analysis. Precoated HPTLC plates which are available in the market are more appropriate for HPTLC analysis. Plastic sheet or aluminum foil supported HPTLC plates are now more popular compared to the glass plate supported HPTLC plate because they can easily cut into desired sizes as well as require less space to keep in the lab but glass is more resistant to heat and chemical reaction compared to the aluminum and plastic sheets. The mean particle size in HPTLC is 5-6µm compare to the 10-12µm in classical TLC. The layer thickness is 100-200µm in HPTLC but 250 µm in TLC. The relative humidity plays a crucial role to reproduce the result. The relative humidity is variable in the laboratory condition. To avoid such variation it is desirable to precondition (saturate the TLC chamber with the vapors of mobile phase) the TLC chamber. Preconditioning is more required with the highly polar mobile phase.
The environment of the lab contains various dirt particles, a vapor of various gases which can be deposited into the HPTLC plate. This impurity can be removed by prewashing the plate. Run the methanol or such other solvent without applying the sample on the HPTLC plate so that all dirt particles or impurities on the HPTLC plate will be collected on the upper edge which can be removed. After prewashing the plate are kept in the oven for 15-20 min at 120°C which is known as conditioning.
Mobile Phase:
The mobile phase used for HPTLC should be of utmost pure. The presence of antioxidants and stabilizers altered the nature of the chemical. They should be kept in proper storage condition. Polarity, viscosity, volatility are some points that affect the chromatographic procedure. The solvent used for the mobile phase is categorized into 8 different classes. The mobile phase should be kept as simple as possible.
Chromatographic Development:
Chromatographic development is another important aspect of HPTLC. Generally, glass development chambers are used for such purposes. The various development chambers which can be used for chromatographic development are:
- Flat bottom chamber
- Twin trough chamber
- Sandwich chamber
- Horizontal chamber
- Automatic development chamber
- Forced flow development chamber
- Automatic multiple development chamber
The twin trough chamber uses less solvent. The linear development of the chromatogram is the best method in which the HPTLC plate is placed vertically in an appropriate chamber. The solvent is run by capillary action.
Detection:
After the development of chromatogram HPTLC plates are dried and evaluated for the following method:
Evaluation by the non-destructive method:
- Direct visual method
- Evaluation under UV light:
Reversible Reaction:
- Iodine Vapour
- Ammonia Vapour
Non Reversible Reaction:
- Fluorescent dye
- pH indicator
- Wetting/ Dipping
- Spraying technique
All the above techniques come under a non-reversible detection technique in which the isolated constituents cannot be recovered and destroyed after detection.
Scanning and Documentation:
After the development of the spot the HPTLC plate is scanned at selected UV regions wavelength and the selected can be measured in the computer in the form of peak and can be compared with standard compound or other constituents.
The obtained band can be converted into the peak. The height and area peak of the peak corresponding to the concentration of isolated constituents. This document can be stored on the computer for further reference.
Make sure you also check our other amazing Article on : Extraction Methods