Extraction Methods: In the extraction process, the animal/plant drug is treated with a particular solvent. The solvent dissolves the medicinally active constituents in itself but animal or plant tissue and other component are not dissolved in the solvent.
OR
Extraction is the process in which the separation of the soluble constituents occurs from insoluble substances either solid or liquid by processing with a specific solvent.
The active constituents from the crude drug can be separated by different separation and extraction methods. In the extraction process, there is a mass transfer process in which the transfer of mass occurs from soluble material like solid to fluid. The different factors which affect the process of mass transfer are temperature, agitation, size reduction, and others. The extraction may be solid, solid-liquid extraction, or liquid-liquid extraction. In the solid separation of the drug, the active constituents of a solid drug are extracted from solid substances. In solid-liquid extraction, the solid drug is extracted from a liquid solvent. In the case of liquid-liquid extraction, liquid solvent is selected to extract the active constituents present in another liquid. Both liquids are immiscible. Some important term uses in the extraction process are
- Menstruum: Solvent or mixture of solvent used for extraction.
- Miscella: Solution with extracted substance.
- Rinsing: Dissolution of extractive substance out of the disintegrated cell.
- Laxative or leaching: Water is used as a solvent for extraction.
- Marc: Residue left after extracting the desired constituents.
The various methods of extraction are:
- Infusion
- Decoction
- Digestion
- Maceration
- Counter current extraction
- Super critical fluid extraction
- Hot continuous extraction (soxhalation)
- Percolation
- Ultra sound extraction
- Steam distillation
- Microwave assisted extraction
1. Infusion
Table of Contents
In the infusion process, the crude drug is left with solvent to soak them properly at room temperature for a particular period with or without intermittent shaking. After the specific period, the filtration will follow which will separate the plant’s debris from the miscella. Various times the plant material has settled down which has separated by decantation and can replace with other solvents. The apparatus used in the infusion process is depending upon the type of the process. It can be a small test tube or flask to a very large industrial vessel. The infusion process is generally followed when active constituents are water-soluble and they are soft so that water can penetrate the tissue. The final volume is not adjusted in infusion. Infusion can be fresh or concentrated. Examples are Infusion of quassia, senna.

2. Decoction
The process is more suitable for vegetable drugs that are heat stable and water-soluble because in this method the drug is boiled for about 15 min.
Take the drug in a vessel add 1000 ml water and cover it
↓
Boil for this for around 15 minutes
↓
Cool it to room temperature
↓
Strain the liquids and press the marc
Fig.2: Flowchart of decoction method
The term decoction is taken from the Latin word decoquere (meaning to boil down) demeans from and coquere means to cook. In the decoction process, the plant drug is first mashed and then it is boiled with water which helps to extract the active constituents from the drugs. Herbal tea and coffee is a good example of decoction.
3. Digestion
Digestion is a modified form of maceration in which the temperature of the menstruum is kept heated at a particular temperature under pressure. This rise in temperature and pressure helps the menstruum to penetrate the drug and extract it completely. The apparatus used for the digestion is the digestor which is generally made up of metal. The drug is placed inside of it with menstruum under specified temperature and pressure and then screw the cover with the help of nuts.
4. Maceration
In the process of maceration, the crude drug is immersed into the bulk of menstruum or solvent for at least 3 days (generally 3-7 days). During this period the menstruum is agitated frequently. The menstruum and container should be kept in the stoppered container. The mixture is then filtered or strained through a net or sieves. Filter almost all the liquids and then press the marc and clarified liquid by decantation after standing or by filtration. The loss of solvent can be adjusted by prescribed extracted juices. Stoppered containers are generally used for maceration so that the loss of solvent by evaporation should be avoided. The drug is allowed to stand for 3-7 days with menstruum so that solvent penetrates the cell more perfectly and gets the time for portioning of active constituents into the solvents. Frequent agitation helps the distribution of active constituents in the entire solvent and prevents the localization of active constituents around the tissue and cell.
The maceration may be of various types like:
- Simple
- Modified
- Multiple
- Kinetic maceration
- Remaceration
Simple and modified Maceration Both can be better understood by their differences. The differences between these are as follows:
Table.1: Comparison of Simple Maceration and Modified Maceration Methods
| Sr. No. | Simple Maceration | Modified Maceration |
| 1 | Use for the organized drugs (bark, root). | Use for unorganized drugs (gum, resin). |
| 2 | Drug and menstruum are shaken occasionally during 3-7 days. | Drug and 80℅ menstrual shaken occasionally during 3-7 days. |
| 3 | Strain the liquid and press the marc. | Decant the liquid and marc is not pressed. |
| 4 | Mix the liquid obtained and clarify. | Filter one liquid and pass the remaining menstruum. |
| 5 | The filtrate is not adjusted to volume. | The filtrate is adjusted to the volume |
| 6 | Here marc is pressed because contains a considerable amount of macerate (organized drug). | Marc is gummy and compact and does not contain any macerate so pressing of marc is not necessary (unorganized drugs); |
Multiple Maceration:
The maceration process is used for the concentrated preparation where the entire menstruum is segregated into two parts (for double maceration) or in three parts (in triple maceration). Each menstruum is used individually for the maceration process. In this process the drug menstruum ratio is low and extraction is performed with less amount of menstruum.
Kinetic Maceration:
Like the simple maceration kinetic maceration is also preceded into the room temperature but the single difference is that the material is under constant motion. The intensity and type of movement play important role in the maceration.
Re-Maceration:
In the re-maceration process part of the solvent is added to the drug. After the filtration process, the residue is treated with the remaining solvent.
Uses of Maceration:
BP permits the preparation of the tincture by maceration methods of following drugs Squill tincture, Catechu tinctures, Senna liquid extract compound Benzoin tincture, and opium tincture.
Mother tinctures of homeopathic Pharmacopoeia are prepared by maceration methods. Maceration is the most widely used method of extraction.
Maceration can be performed easily with a pharmacist lab with a small amount of sample. Several drugs can extract by only maceration methods due to their high swelling property or high mucilage content.
Disadvantages:
Maceration is not an exhaustive extraction process. This factor becomes more significant when the drug is expensive. It is a batch process.
5. Percolation
The word percolation refers to the “First pass through”. Percolation can be defined as ‘Short successive maceration or extraction by the method of displacement’. Percolation can be performed into the conical percolator, cylindrical percolator, or steam jacketed percolator. The various stages involved in the percolation are following:
(a) Size reduction: To assure the complete exhaustion of the crude drug, the drug should be suitable size reduced. Size reduction also increases the surface area of the crude drug and more surface area of the crude drug will be available to react with the menstruum. Size reduction is also helpful for uniform packing of the crude drug in a percolator and reducing the moment of menstruum in the percolator.
(b) Imbibition: Imbibition is a process in which the powdered drug is kept along with menstruum for 4 hours in a well-stoppered container. During this duration, the menstruum penetrates the cell wall. This initial moistening of the crude drug powder is very important because it reduces the chances of choking of percolator though the dried drug swells when comes into contact with menstruum. The swelling of the drug reduces the porosity of the powder and chokes the percolator. Imbibitions replace the interstices air which can otherwise affect the flow of menstruum. Imbibitions also prevent the washing of fine particles of the crude drug during percolation.
(c) Packing: After imbibition, the lump of the crude drug must be broken. The lower end of the percolator should be plucked with cotton and then place the drug powder layer by layer. The packing should be perfect, neither too tight nor too loose which may affect the flow of menstruum. Two third of the percolator should be covered with the drug on which place the piece of filter paper and wash sand should be placed on the top of the filter paper. This prevents any type of disturbance of the crude drug by the flow of menstruum.
(d) Maceration: Sufficient amount of menstruum should be added after packing the percolator. During the addition of menstruum, the lower tap of the percolator should be open so that air present in the percolator is displaced by the menstruum. When menstruum starts to come from the lower tap close the tap and allow it to stand, the percolator for 24 hours as the primary maceration takes place. The menstruum level should be above the drug bed.
(e) Percolation: Open the lower tap after 24 hours of maceration and collect the menstruum from the lower end until the three fourth portion of the final product is obtained. Meanwhile, sufficient menstruum is added over the powdered drug so that the packed drug does not become dry. Completion of the percolation process should be checked by various methods like:
- By checking the specific gravity of the end few ml of percolate and compare with the specific gravity of fresh menstruum.
- Evaporate the last few ml of percolate to dryness and see if any residue comes after evaporation or not.
- Perform the specific chemical test for the last few ml of percolate like tannins, glycoside, alkaloids, etc whether it is positive or negative.
(f) Pressing the marc: In the last, the marc should be pressed, and obtain liquid is added into the collected menstruum. More menstruum should be added to obtain the desired volume. Allow the liquid to stand and separate the suspended particles by filtration or decantation. Example- Strong tincture of ginger, tincture of belladonna, etc.
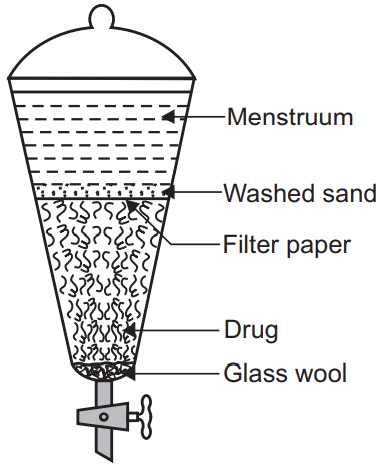
Reserved Percolation:
In this type of percolation, the initial portion (near about three fourth of the percentage of menstruum) which have the major quantity of active ingredient is kept separate just like the simple percolation process and the last one-fourth amount of menstruum is collected in another vessel and concentrated by the method of evaporation. This concentrated syrupy fluid should be added into the previous three fourth portion of menstruum. Adjust the final volume. The last one-fourth portions contain very less amount active ingredients so, with the help of evaporation generally, we concentrate the menstruum. Example- Liquid extract of licorice.
The process has the advantage that we obtain the concentrated menstruum without heating the major portion (three fourth portions) which have more active ingredient and just heat the last one-fourth of portions which also save energy as well as handles less amount fluid for the process.
Modified Percolation:
In the modified percolation process we reduce the drug/percolate ratio to 1:3 which is generally 1:4 in the common percolation process. By this, we can save menstruum, time, and heat. Percolation is a type of displacement method. It has been observed that a stationary menstruum dissolves the more active constituents because stationary menstruum may remain in contact with more time to drug compare to the moving menstruum. Hence more quantity of menstruum is required to exhaust the drug in the case of a simple percolation process. But if a percolation process is broken into the various step of maceration the drug/ product ratio will be reduced to 1:3.
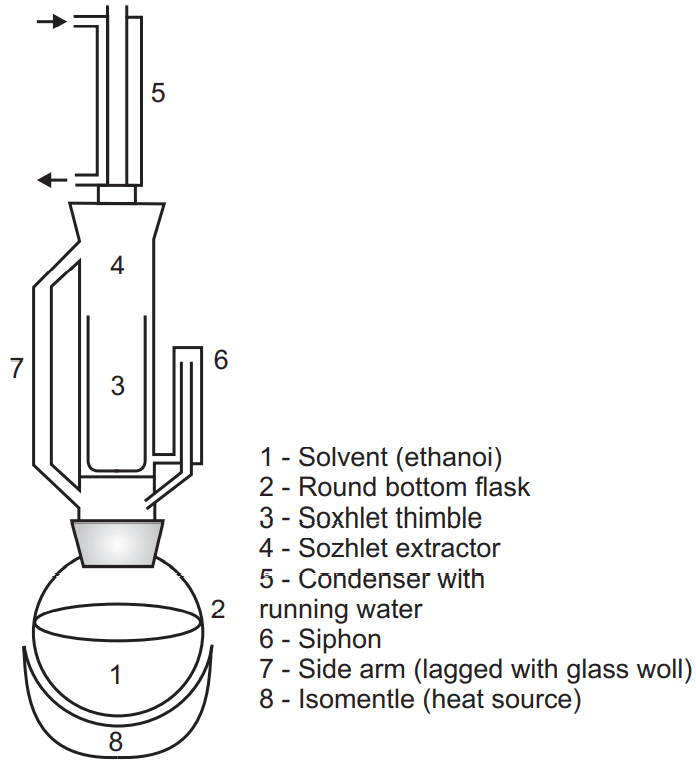
Soxhlet Extraction/Continuous Hot Percolation Process:
This extraction process is more suitable where menstruum has less penetration into the cellular tissue. Active constituents are not readily extracted with a solvent. The quantity of solvent for extraction of the active constituents is very less.
Soxhlet extraction is preferable in the above-mentioned conditions. Here the hot menstruum cycle is passed again and again over the crude drug and solubilizes its active ingredient until the drug becomes exhausted. The apparatus consist of three important parts:
- A round bottom flask (RBF) contains menstruum.
- Extraction chamber in which drug is kept in a thimble.
- Condenser.
The size reduction of the crude drug was performed and packed the size reduced drug was into the thimble (made of filter paper or cellulose) which is helpful to prevent choking of the extractor.
Place the solvent in the round bottom flask (RBF) and boil it by the heating mantle. The vapor of the solvent goes to the condenser via a side tube. The condenser condenses the vapor of solvent and this solvent penetrates the packed drug. It percolates the crude drug and extracted the active ingredient. The level of menstruum is increased in the extractor until it reaches to maximum point from where it is siphoned into the round bottom flask.
This menstruum is again heated in RBF where active constituent remains in RBF and vaporized solvent again passes through the side tube and condenser which penetrates the drug present in the extractor. The process will continue till the drug become exhausted. Some drug-like resin, opium, gum blocks the Soxhlet apparatus so this is not a suitable method for these drugs.
Thermolabile drugs and constituents are not suitably extracted by this method because the active ingredient can be destroyed. Pure solvent or only those solvent mixtures which have constant boiling temperature can be used for extraction purposes.
6. Microwave-Assisted Extraction
The electromagnetic radiation which has a frequency of 0.3-300 GHz is called microwave. Industrial microwaves are generally operated at 2.45 GHz so that they avoid the interferences of domestic instruments like radio and others. Due to their electromagnetic property microwaves contain magnetic and electric fields. They are perpendicular to one another. The electric field generates heat through two simultaneous methods i.e. ionic conduction and dipolar rotation. Microwaves transfer the energy to solid matrix and solvent homogenously and very efficiently. Substance (solid matrix and solvent) absorb the energy as per the dielectric constant. The plant material which is present in the microwave transport solvent absorbs the heat of the microwave which causes the heating of moisture present inside the drug. Evaporation occurs due to the heating of moisture and this will produce high vapor pressure. This high vapor pressure cracks the cell wall of the plant drug and releases the active constituent into the solvent. The extraction property of the solvent and its interaction with a microwave can be altered by using solvent mixtures.
The benefit of microwave-assisted extraction is that fewer solvents are required, time consumption is less, high extraction rate and good reproducibility but additional centrifugation or filtration are necessary to remove residue and the efficiency will be poor if the solvent or compound have non-polar property or they are volatile. Example- Extract glycyrrhizic acid from mulethi.
7. Ultrasonic Assisted Extraction
The frequencies above 20,000 Hz are known as ultrasound. Ultrasonic waves are used in ultrasonic extraction. These waves cause cavitations to affect the dry cell and destruct the cell wall and release the active constituents. When ultrasonic waves are passed through the liquid media it compresses (produce high pressure) and rarefaction (low pressure) to the liquid media. Due to this process, small voids or vacuum bubbles are formed in the solvent. After a certain duration, these bubbles are not able to absorb more energy produced by the microwave and they busted. At the high-pressure cycle, they busted which is known as cavitation. Due to this cavitation cell walls are destructed and active chemical constituents are extracted.
This process is efficient, inexpensive, and simply compare to conventional extraction techniques. It has a high yield, reduces operating temperature, and is easy to operate but sometimes active constituents can convert into free radicals which have undesirable side effects.
8. Steam Distillation
It is one of the most popular methods to separate the essential oil from crude drugs. A still (large stainless steel container) contain the crude drug material. The steam is injected through the inlet valve into the plant material which contains the essential oil. The inlet steel evaporates the volatile molecule. This volatile molecule is collected into the condenser. The water molecule and volatile oil molecule can be easily separated because they are not mixable. Direct prolonged heating should be avoided which deteriorates the volatile components.
9. Counter Current Extraction
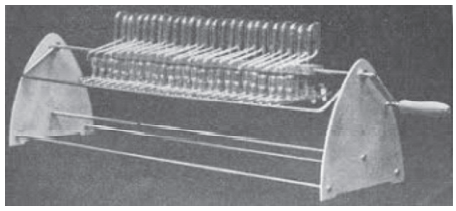
In counter-current extraction, the substance which is to be extracted is distributed between two different solvents depending upon their distribution coefficient. This can be best performed by Craig apparatus. There are several glass tubes (r = 0, 1, 2, 3, —-) in the Craig apparatus and they are designed such that the solvent which is lighter in density can be transferred from the first tube to the second tube and then forward. The tubes are driven or shaken electromechanically. The lower phase which has a higher density is called as stationary phase and the solvent which has the lighter density is called the mobile phase. The substance to be extracted is present in the stationary phase. An equal quantity of stationary phase is present in all the tubes.
The mobile phase (solvent has less density) is transferred into the first tube (tube =0) shake properly so that extraction takes place and then phases are allowed to separate down. The mobile phase (or upper phase) is then transferred to the next tube no 1and repeat the same process and transfer it into further tube-like tubes no 2, 3, 4, and so on after every shaking at the fresh mobile phase to tube no 0.
The substance having a higher distribution ratio separates more quickly compared to the lower distribution ratio. Now a day’s Craig apparatus is less frequently used because of the availability of more recent chromatographic techniques but it has more significance to clear the concept of extraction to the students. It is very difficult to construct and operate a Craig apparatus having more than 100 test tubes.
10. Super Critical Fluid Extraction
A critical point is a particular temperature and pressure at which two phases (like liquid and vapor) can co-exist. The substance above the critical temperature and pressure or the critical point is known as a supercritical fluid. The property of the supercritical fluid is in between gas and pure liquid so that they are also known as dense gases or compressible liquid. Supercritical fluid has good solvation power, low viscosity, higher diffusibility, and good penetrating power. With the help of a supercritical fluid extraction technique, we can separate the constituents from the drug with the help of supercritical fluid. Generally, we can separate the constituents from the solid matrix but it may also be liquid. CO2 is one of the most common supercritical fluids. To make CO2 supercritical fluid the critical temperature is 31°C and the critical pressure is 74 bars.
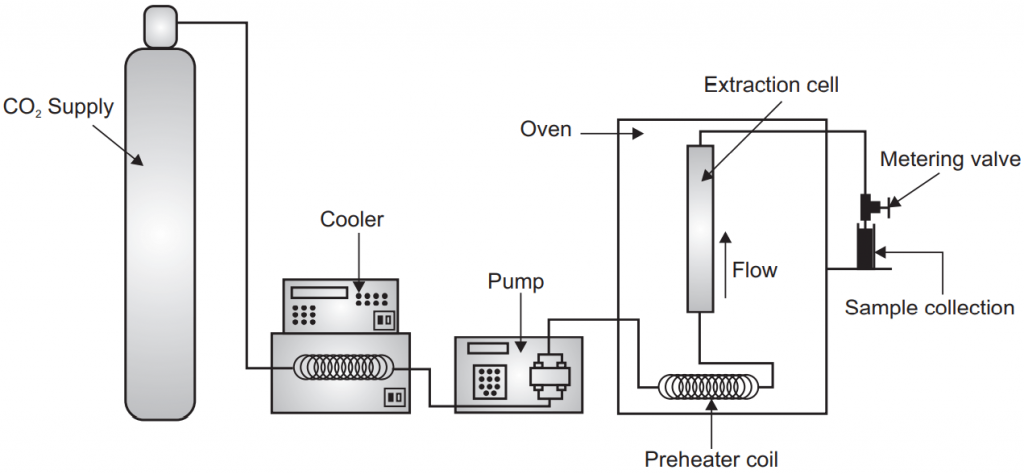
Instrumentation:
The different component in the supercritical fluid extraction is the first one is a fluid reservoir which contains the fluid-like gas cylinder in the case of CO2. Another one is the pump; the pump may be a syringe pump or reciprocating pump. Another important is the extractor which may be made of stainless steel and can with a stand to high pressure like 300 to 600 atmosphere. Another important component of the supercritical fluid extractor is the restrictor. It is important for the controlled and systematic release of the pressure inside the extractor vesicle. The restrictor may be a fixed type or variable type. The isolated constituent is collected in a collector where it can be detected with the help of a detector. Sometimes modifiers are also added into the supercritical fluid to increase its versatility like one to ten percent of methanol is added in CO2.
During the process the liquid is pumped into a heating zone and heated to supercritical temperature then the supercritical fluid passes through the extraction vessel where it diffuses to the solid matrix and dissolves the active constituents. The extracted material with supercritical fluid comes into the separator or collector unit where the pressure is low. The extracted material settles down here and the CO2 can then be cooled, recycled, or discharge into the environment. The isolated constituent can be detected with the help of a detector.
Advantages and Limitations:
The supercritical fluid extraction technique is very rapid and there is no need for organic solvent for the extraction purpose. The thermolabile substance can also be extracted with this technique. It is a versatile and efficient continuous method with complete separation and recovery of the solvent.
Sometimes its consistency and reproducibility may vary. It is an expensive technique because it needs high pressure which increases its cost. CO2 cannot always be used as a solvent as it is non-polar and so limited dissolving power particularly in the case of a polar solvent.
Make sure you also check our other amazing Article on : Vincristine And Vinblastine