Aim: To perform the conductometric titration of strong acid against a strong base.
Principle of Conductometric titrations: The principle of conductometric titration is based on the fact that during titration, one of the ions is replaced by the other and invariably these two ions differ in the ionic conductivity with the result that the conductivity of the solution varies during titration. The equivalence point may be located graphically by plotting the change in conductance as a function of the volume of titrant added. To reduce the influence of errors in the conductometric titration to a minimum, the angle between the two branches of the titration curve should be as small as possible. If the angle is very obtuse, a small error in the conductance data can cause a large deviation. The following approximate rules will be found useful.
1. The smaller the conductivity of the ion which replaces the reacting ion, the more accurate will be the result. Thus it is preferable to titrate a silver salt with lithium chloride rather than with HCl. Generally, cations should be titrated with lithium salts and anions with acetates as these ions have low conductivity.
2. The larger the conductivity of the anion of the reagent which reacts with the cation to be determined, or vice versa, the more acute is the angle of the titration curve.
3. The titration of slightly iodized salt does not give good results, since the conductivity increases continuously from the commencement. Hence, the salt present in the cell should be virtually completely dissociated; for a similar reason; the added reagent should also be a strong electrolyte.
4. Throughout a titration the volume of the solution is always increasing, unless the conductance is corrected for this effect, non-linear titration curves result. The correction can be accomplished by multiplying the observed conductance either by total volume (V V’) or by the factor (V V’)/V, where V is the initial volume of solution and V’ is the total volume of the reagent added. The correction presupposes that the conductivity is a linear function of dilution, this is true only to a first approximation.
5. In the interest of keeping V small, the reagent for the conductometric titration is ordinarily several times more concentrated than the solution being titrated (at least 10-20 times). A micro burette may then be used for the volumetric measurement. The main advantages of conductometric titration are its applicability to very dilute, and colored solutions and to a system that involves relatively incomplete reactions. For example, in which neither a potentiometric, nor indicator method can be used for the neutralization titration of phenol (Ka = 10–10), a conductometric endpoint can be successfully applied.
Application: Acid-base titration, especially at trace levels. Relative precision is better than 1% at all levels. There are also a few disadvantages to this technique. As you know conductance is a non-specific property, concentration of other electrolytes can be troublesome. The electrical conductance of a solution is a measure of its current carrying capacity and is therefore determined by the total ionic strength. It is a non-specific property and for this reason, direct conductance measurements are of little use unless the solution contains only the electrolyte to be determined or the concentrations of other ionic species in the solution are known. Conductometric titrations, in which the species in the solution are converted to non-ionic for neutralization, precipitation, etc. are of more value.
Procedure: Take 50 ml of 0.1 M HCl. Add ml wise 0.1 M NaOH solution in an acid-containing flask. After the addition of NaOH, measure the conductance by conductometry.
Plot a graph of ml of NaOH Vs conductance and locate the equivalent point.
Observation Table
| Sr.No. | ml of NaOH (B.R) | Conductance |
Graph:
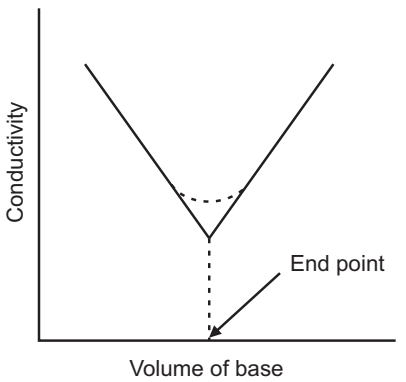
Result: The normality of sodium hydroxide is …… N.
Make sure you also check our other amazing Article on : Precipitation Titration
