Aim: To determine the volume of a strong acid (HCl) and weak acid (CH3COOH) in the given unknown solution and to estimate the amount of HCl and CH3COOH present in 100 ml of the given mixture of the acid solution by titrating against a strong base (NaOH), conductometrically.
Theory: Conductometric titrations work on the principle of Ohm’s law. As current (I) is inversely proportional to resistance (R) and the reciprocal of resistance is termed conductance, and its unit is Siemen (mho) cm−1, the electrical conductivity of a solution depends on the number of ions and their mobility. Ionic interactions may occur when one electrolytic solution is added to another electrolytic solution, resulting in changes in the conductivity of the solution. For example, in the titration of HCl and CH3COOH versus NaOH, the addition of a strong base (NaOH) to a strong acid (HCl) and weak acid (CH3COOH) would cause changes in the conductivity of the solution, due to the replacement of the highly mobile hydrogen ions (H+) by the less mobile sodium ions (Na+).
Thus, the principle of conductometric titrations is the substitution of ions of one mobility by ions of another mobility. In conductometric titrations, the titrant is added from the burette, and the conductivity readings (corresponding to various increments of titrant) are plotted against the volume of the titrant. Upon adding a strong base to the mixture of acids, the conductance falls until the strong acid is neutralized then raised as weak acid is converted into its salt, and finally raises more steps as the excess alkali is introduced. Such a titration curve consists of 3 lines that intersect at a particular point, known as the endpoint or equivalence point.
In this experiment, the base (NaOH) will be titrated against the acid (HCl), and the change in the conductivity of the solution will be measured conductometrically. Consider that the acid (HCl) is taken in the beaker, while the base (NaOH) is taken in the burette. The solution in the beaker (HCl) at first contains H+ and Cl− ions. After that, when a small amount of NaOH is added to CH3COOH, the conductivity decreases slowly. Since the concentration of H+ ions in CH3COOH is small and H+ ions possess the greatest mobility, the conductivity of the solution increases gradually due to the formation of Na+ and CH3COO− ions. It follows that the conductivity of this solution is mainly due to H+ ions.
The addition of NaOH is represented by:
H+ + Cl− + Na+ + OH− → Na+ + Cl− + H2O
CH3COO− + H+ + Na+ + OH− → CH3COO− + Na+ + H2O
As NaOH is added, the H+ ions are removed as ionized water. Therefore, the conductivity will decrease, since Na+ ions do not possess much mobility. At the neutralization point, the solution contains Na+ and Cl− ions and will have considerably less conductivity than the original value. If 1 drop of NaOH is added, after the neutralization point, there will be a small concentration of OH− ions. Therefore, the conductivity increases, as OH− ions have the second-highest mobility. As more and more NaOH is added, the conductivity keeps on increasing continuously. Hence, on plotting the corrected conductivity values as ordinate (y-axis) against the volume of titrant added as abscissa (x-axis), we get two straight lines, and the point of intersection gives the equivalence point.
Materials required: Conductivity meter (with cell), burette (50 ml), volumetric flask (100 ml), conical flask (100 ml), pipette (10 ml, 25 ml), beakers (100 ml), stirrer/ glass rod, oxalic acid solution (0.1 N), HCl solution, CH3COOH solution, NaOH solution.
Procedure: Part-I: Standardization of NaOH against standard oxalic acid (0.1 N)
- 10 ml of given 0.1 N standard oxalic acid is pipetted out into a 100 ml conical flask.
- This solution is titrated against the given unknown concentration of NaOH using 1-2 drops of phenolphthalein indicator until the endpoint is colorless to pale pink.
- Tabulate the values and repeat the titration for concurrent readings and determine the concentration of supplied NaOH solution.
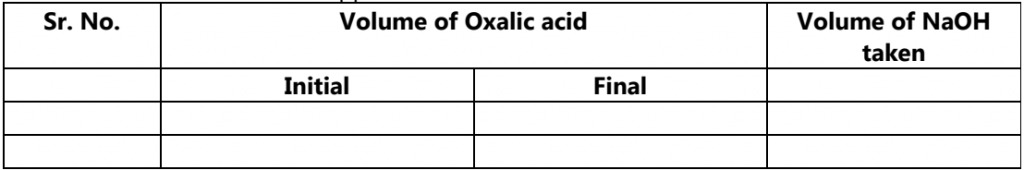
(HCOO)2 N1V1 = N2V2(NaOH)
Part-II: Measurement of Conductivity
- The conductivity meter is calibrated or standardized by using a standard 0.1 N KCl solution.
- 25 ml of given HCl and 25 ml of given CH3COOH solution are pipetted out in a clean beaker and then it is made up to 100 ml by adding 50 ml of distilled water.
- Now, the conductivity cell is immersed in the beaker and the initial conductance of the solution is taken by stirring the solution and keeping it constant.
- Then, 0.5 ml portions of NaOH are added from the burette and stirred well. The conductance of the solution for each addition is to be noted.
- The conductance of the solution decreases till the equivalence point of a strong acid is observed.
- After the equivalence point, on continuing the addition of NaOH there will be a small rise in conductance values till the endpoint of a weak acid is reached.
- After that, the conductivity value increases suddenly due to the conductance of OH− ions.
- Plot the graph concerning the volume of NaOH consumed versus corrected conductance. The intersection point on the graph which gives 2 minima of the conductance value represents the equivalence points of HCl and CH3COOH.
- The conductivity is corrected by multiplying with the factor [(V + V)/V], where ‘v’ is the volume of base added and ‘V’ is the volume of solution initially taken in the beaker.
- From the known solution, calculate the strength and amount of HCl and CH3COOH present in 100 ml of the solution.
- From the unknown solution, calculate the volume of HCl and CH3COOH present in the solution.
- Theoretical and Graphical calculations are to be shown in the separate and proper format for the known and unknown solutions with all formulas, units, results, etc.
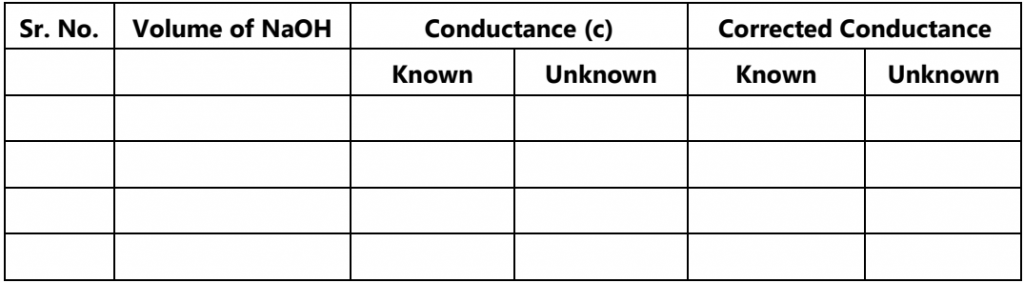
(NaOH) N2V2 = N3V3 (HCl)
(NaOH) N2V2 = N4V4 (CH3COOH)
Observations and Calculations: Room Temperature = 0°C.
- The values of the corrected conductivity are plotted as ordinate (y-axis) against the volume of NaOH solution added as abscissa (x-axis).
- The point of intersection of the 3 curves gives the volume of NaOH solution at the equivalence point.
- The volumes of the unknown acid solution and the amount of HCl and CH3COOH present in 100 ml of the supplied solution are to be calculated.
Note: Handle the instrument, electrodes (cell), and glassware with care. Avoid parallax errors while taking the readings. The electrode should not touch the walls of the beaker.
Results:
- Strength of NaOH solution = …….
- Strength of acid HCl = …….
- Strength of CH3COOH =…….
- The volume of unknown acid mixture solution (HCl and CH3COOH) =…….
- Amount of HCl and CH3COOH present in 100 ml of the original solution = …….
- Comment on the nature of the graph(s).
Make sure you also check our other amazing Article on : Assay of Sodium Chloride