Granulation Process:
Table of Contents
A manufacturing method must also be determined once a tablet formulation is designed with a drug, binder, disintegrant, diluent, pH buffer, lubricant, or a matrix former polymer.
The manufacturing method will depend on the dose of the active ingredient, the limitations of drug substance such as heat sensitivity or water insolubility, availability of specialized equipment such as high-shear granulators or fluidized beds, the time frame for manufacturing, and the batch size. Certain manufacturing methods can be used interchangeably. On the other hand, sometimes a specific manufacturing method must be employed, for instance, passing the formulation three times through a roller compactor and compressing a 1500-mg formulation to produce a tablet of reasonable size.

Therefore, deciding on a manufacturing method is a complex task that requires time, equipment, formulation optimization, and close collaboration between formulation scientists and process engineers. In general terms, there are three manufacturing processes or Granulation Methods for tablets:
(1) wet granulation
(2) dry granulation
(3) direct compression as discussed below.
Wet Granulation – [Aqueous Granulation Technique]
The purpose of wet granulation is to convert the drug and excipient mixture into granules that flow well into dies, and which are compressible into mechanically strong and acceptable tablets. Wet granulation has many subtypes.
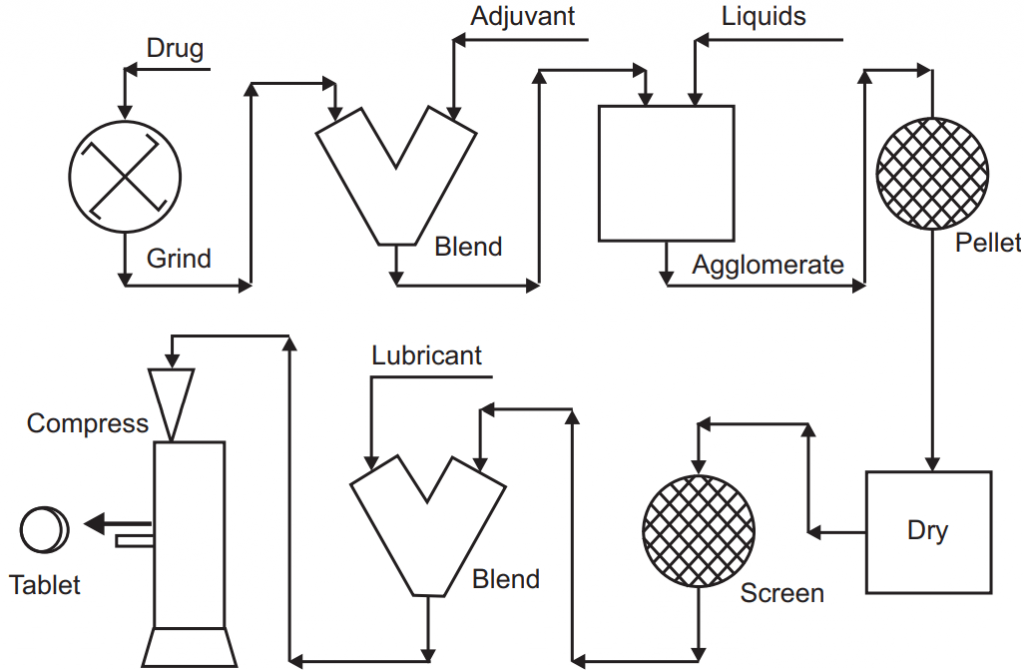
The classical traditional wet granulation operation consists of the following processes:
- Premixing drug with other ingredients using a V-shaped mixer.
- Transferring the mixture into a traditional low shear granulator where a binder solution is added under a mechanical shear until a certain granule size and binding are obtained.
- Wet sieving of granules through the desired screen size.
- Drying of granules in a tray-oven dryer.
- Dry sieving/milling of granules to a certain particle size distribution.
- Adding a lubricant to the dry granules.
- Compressing the granules into tablets.
The wet granulation operation as described above has been used for more than 50 years in the pharmaceutical industry, and there is great expertise in this area. However, the introduction of new materials and equipment, changing manufacturing needs, and time limitations for manufacturing have brought new modifications to classical wet granulation. Two different modified wet granulation operations are summarized below:
Modification to traditional wet granulation involves the following:
- Premixing drug with other ingredients using a V-shaped mixer or a high-shear mixer/granulator
- Transferring the mixture into a high-shear granulator where a binder solution is added, and certain granule size is obtained in a very short time.
- Wet sieving of granules through the desired screen size may or may not be required because of very uniform and narrow particle size distribution.
- Drying of granules in a fluidized bed or a microwave dryer.
- Alternatively, drying the wet mass in the same high-shear mixer/granulator.
- Dry sieving/milling of granules to a certain particle size distribution.
- Adding a lubricant to dry granules.
- Compressing the granules into tablets.
Modification to traditional wet granulation-II involves the following:
- Premixing drug with other ingredients using a V-shaped mixer.
- Transferring the mixture into a fluidized-bed granulator where a binder solution is sprayed until a certain granule size and simultaneous drying occur.
- Dry sieving/milling of granules to a certain particle size distribution.
- Adding a lubricant to dry granules.
- Compressing the granules into tablets.
Based on the modifications summarized above, traditional wet granulation has become a more feasible, time-saving, and economical operation. In a regular workday, many batches can be manufactured with high reproducibility. The high-shear granulators and/or fluidized-bed granulators/dryers are at the center of these operations. Among the advantages of wet granulation is better drug content uniformity for low-dose drugs, better mechanical tablet properties such as high crushing strength, and low friability due to well-distributed binder molecules over the drug and diluent particles. Expensive equipment, limited batch capacity, and the need for drying are among the disadvantages of wet granulation operations.
Dry Granulation – [Non-Aqueous Granulation Technique]
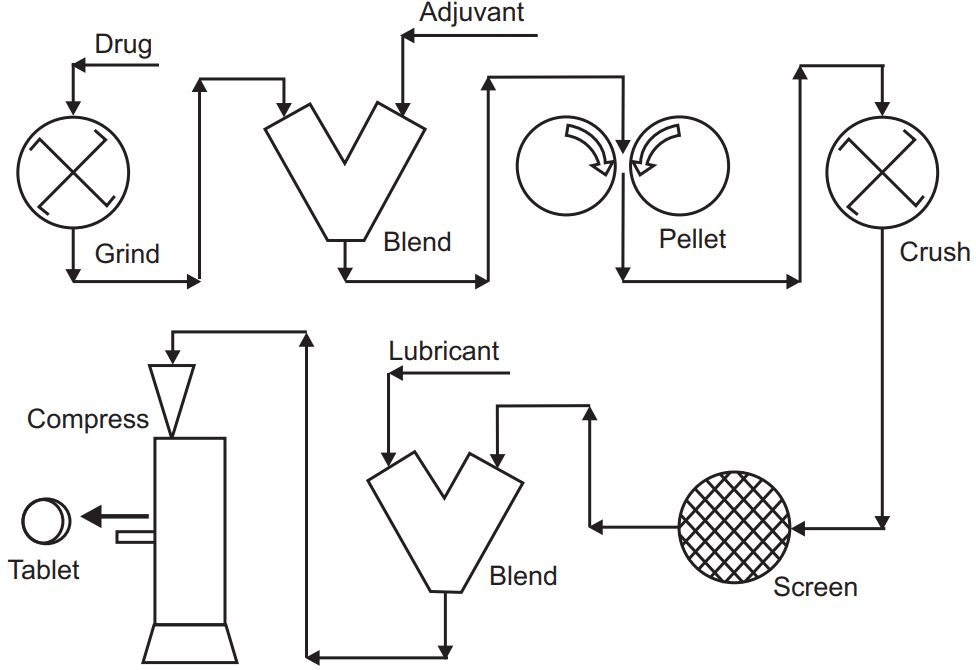
Dry granulation is the method of producing granules without any solvent use or drying before tablet manufacturing. The flow and compressibility are attained by mechanical compression. A dry binder such as MCC or a polymer such as HPMC that will contribute to plastic deformation can be added to the mixture. In the past, before the availability of continuous roller compactors, compressing briquette tablets in specialized tablet presses and breaking those tablets into granules was the method of choice, but this operation produced dust and had other problems. Therefore, dry granulation was not a preferred way of manufacturing tablets. In the modern application of dry granulation, counter-rotating steel rollers are used to apply pressure to the powder. The surface of these rollers can be smooth or grooved depending on the properties of the powder. The compressed product has a higher density upon processing between the rollers. Some formulations may require a double or triple pass-through roller compactor for maximum densification. The final product will require size reduction and sieving. Modern dry granulation using roller compactors is a straightforward, reproducible operation with few steps. Hence, it is a rapid and economical way of producing pharmaceutical granules. The selection and concentration of formulation ingredients as well as their mechanical properties are more critical in this process than in wet granulation.
Basic steps in Dry Granulation are:
- Premixing drug with other ingredients using a V-shaped mixer.
- Transferring the mixture into a roller compactor using closed-circuit vacuum equipment to prevent dust formation.
- Milling/sieving of the resulting flakes or ribbons into granules.
- Adding lubricant and/or disintegrant to granules, and finally.
- Compressing the granules into tablets.
The operation parameters that affect the product properties as might be anticipated include roll pressure, speed, surface texture, and the gap between the rollers. The powders are fed to a roller compactor by a screw feeder under positive pressure. For those tablets that approach or exceed 1000 mg such as the 825 mg amoxicillin and 125 mg potassium clavulanate combination, dry granulation is the most feasible way of manufacture.
Direct Compression
As the name implies, this method involves no further processing of powders before tableting. A formulation is well mixed to ensure uniform drug distribution, and after adding a lubricant, tablets are compressed. However, the requirements for flowability and compressibility must be met by the excipients or the drug substance requires to be coated or processed. Since direct compression involves only mixing and compressing steps, it is the most preferred way of making tablets, given the appropriate choice of excipients.
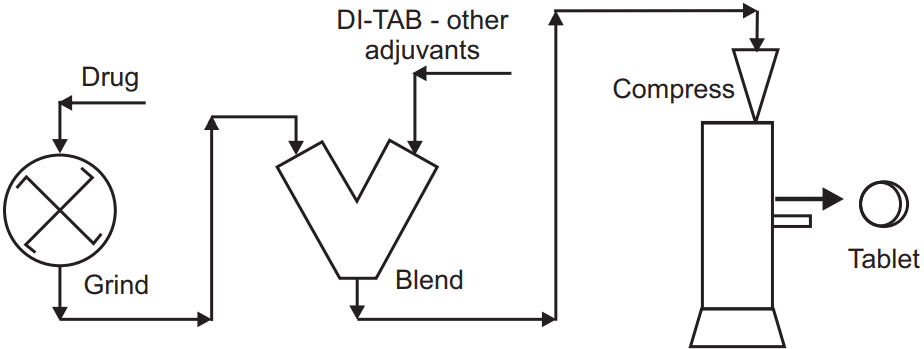
Steps in Direct Compression are:
- Premixing drug with other ingredients using a V-shaped mixer
- Adding a lubricant to the granules
- Mixing with lubricant 3 to10 minutes
- Compressing the granules
Availability of DC excipients does not guarantee the success of DC tableting. The drug substance must also have appropriate compressibility and flowability if the drug represents more than one-third of the tablet formulation. In that case, DC excipients may compensate for flowability and compressibility. If a drug powder with an insufficient flow and compressibility forms a large portion of a tablet, the DC method cannot be used.
Active materials such as acetaminophen, amoxicillin, ascorbic acid, thiamine, and riboflavin have DC grades. The DC grades are produced by coprocessing the actives with polymers such as MC, hydroxypropyl MC, acrylates, or PVP through a spray-drying or fluidized-bed coating process. Direct compression requires directly compressible excipients, thus limiting a formulator’s ability for further processing.
However, once a compressible formulation is designed, manufacturing is straightforward. A pharmaceutical company that manufactures tablets only with the DC method needs only powder-powder mixers and tablet presses, which means lower investment costs. MCC is the most important DC excipient. Other DC excipients include anhydrous b-lactose, spray-dried lactose, unmilled dicalcium phosphate dihydrate, pregelatinized starch, and mannitol DC.
Make sure you also check our other amazing Article on : Parenteral Dosage Form