The ionized form and unionized form or the different resonant forms of indicators generally apply equally well for non-aqueous titrations but their colour changes at the endpoint vary from titration to titration, as they depend on the nature of the titrant.
The endpoint may be established by carrying out a potentiometric titration while simultaneously observing the colour change of the indicator.
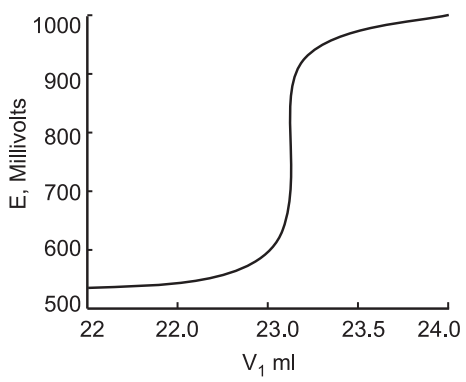
The appropriate equivalence point can be determined from the inflexion point of the titration curve.
The majority of non-aqueous titrations are carried out using a fairly limited range of indicators, some typical examples are given:
1. Crystal Violet: Used as 0.5% w/v solution in glacial acetic acid. In reactions such as pyridine are titrated with perchloric acid, its colour change from violet through blue followed by green, then to greenish yellow.
2. Methyl Red: Used as a 0.2% w/v solution in dioxane, gives a colour change from yellow to red.
3. Naphthol Benzein: Used as a 0.2% w/v solution in acetic acid, gives a colour change from yellow to green. It gives sharp end points in nitromethane containing acetic anhydride for titration of weak bases against perchloric acid.
4. Quenaldine Red: Used as a 0.1% w/v solution in ethanol, gives a colour change from purple red to pale green. It is generally used for determinations of drugs in dimethylformamide solution.
5. Thymol Blue: Used as a 0.2% w/v solution in methanol, gives a sharp colour change from yellow to blue at the endpoint. Used extensively as an indicator for titrations of substances acting as acids in dimethyl formamide solution.
Make sure you also check our other amazing Article on : Examples of Non Aqueous Solvents
