Molecular Formula: C9H13NO3
Molecular Weight: 183.2
In general, the reaction taking place between a primary amine and perchloric acid may be expressed as follows:
R-NH2 + HClO4 → [R-NH3]+ + ClO−4
Material Required: Adrenaline 0.2 g; glacial acetic acid: 50 mL; 0.1 N perchloric acid and crystal violet or oracet blue B solution.
Procedure: Weigh accurately about 0.3 g of adrenaline into a 250 mL conical flask; add glacial acetic acid (50 mL), and warm gently, if necessary. Cool and titrate with 0.1 N perchloric acid using crystal violet or oracet blue B as an indicator.
Reaction:
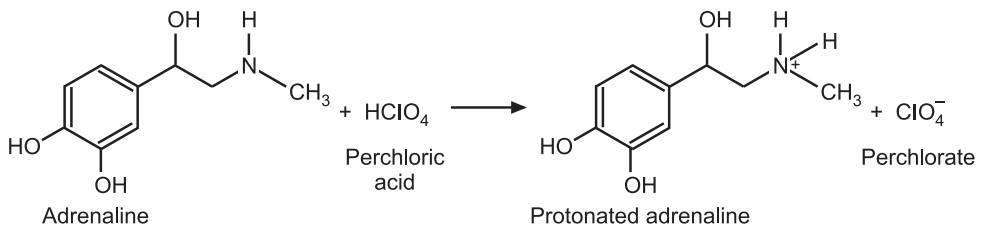
Factor Calculation:
1 mole of perchloric acid ≅ 1 mole of adrenaline
1000 mL of 1N HClO4 ≅ 183.2 g of C9H13NO3
1 mL of 0.1N HClO4 ≅ 0.01832 g of C9H13NO3
Make sure you also check our other amazing Article on : Examples of Non Aqueous Solvents
