All amphiprotic substances are also amphoteric, but the reverse is not true. There are amphoteric substances that do not either donate or accept hydrogen ions when they act as acids or bases. There is a whole new definition of acid-base behaviour which does not necessarily involve hydrogen ions at all. In 1938, Gilbert N. Lewis proposed the acid-base theory on the basis of electron pair acceptance and donation,
According to this theory,
An acid is a substance that accepts an electron pair. (Example: HCl)
A base is a substance that donates an electron pair. (Example: NH3)
Examples of Lewis’s Reactions
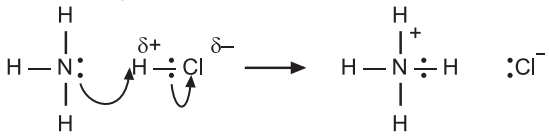
1. Between HCl and NH3: Hydrochloric acid (HCl) is a Lewis acid as it can accept an electron pair. Ammonia molecule (: NH3) has an electron-pair which it can donate and is a Lewis base.
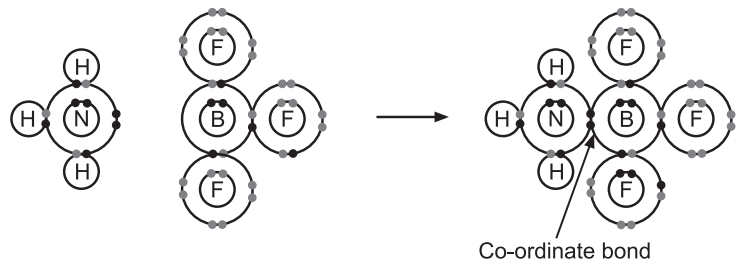
2. Between BF3 and NH3: BF3 has six valence electrons with B atom which can accept an electron pair and is a Lewis acid. The N atom of NH3 has a lone electron pair and is a Lewis base.
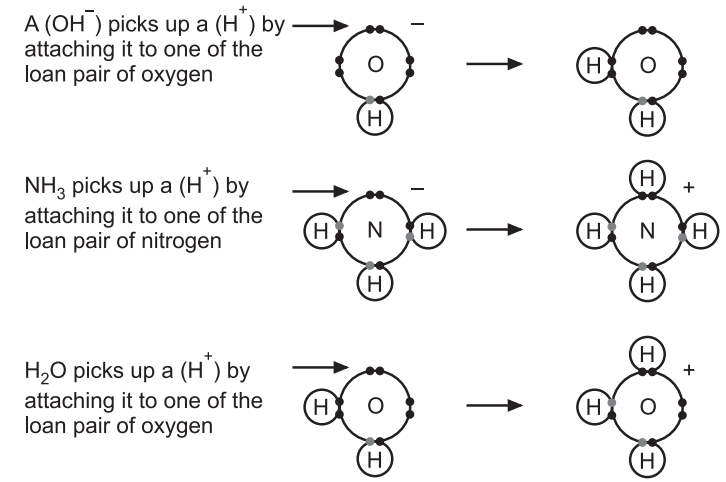
The Relationship Between the Lewis Theory and the Bronsted-Lowry Theory:
According to Bronsted-Lowry hydroxide ions, ammonia, and water base because they are combining with hydrogen ions (H+) with co-ordinate bonding. The reason they are combining with hydrogen ions (H+) is that they have lone pairs of electrons which is in accordance with the Lewis theory.
Advantages of Lewis Theory
1. Lewis acid-base titration can be carried out in a variety of solvents which is restricted in Bronsted-Lowry theory.
2. Co-ordinate bond formation during neutralization of acid and base.
Limitation of Lewis Theory
The relative strength of acids and bases can not be explained by this concept.
Make sure you also check our other amazing Article on : Definition of Acid Base Titration