Svante Arrhenius in 1887 proposed the first theory describing acids and bases. According to this theory.
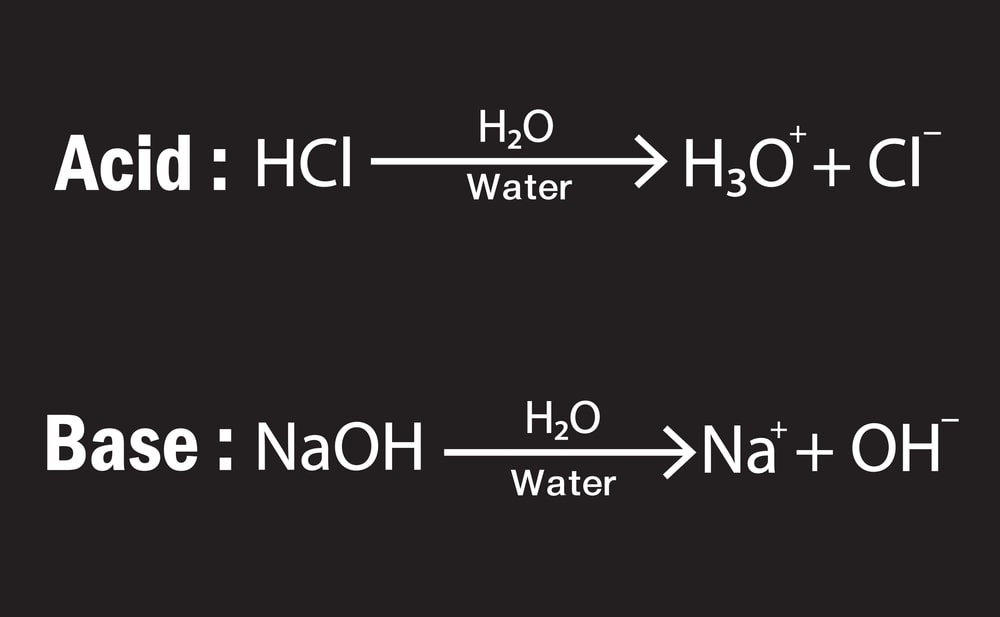
An acid is a substance that dissociates in water to produce one or more hydrogen ions (H+).
A base is a substance that dissociates in water to produce one or more hydroxide ions (OH−).
Neutralization happens because hydrogen ions and hydroxide ions react to produce water.
H+(aq) + OH−(aq) → H2O(l)
Limitations of Arrhenius Theory
1. Some compounds are basic in nature but they do not contain hydroxyl group (OH–) in their structure.
Example: Hydrochloric acid is neutralized by both sodium hydroxide solution and ammonia solution. In both cases, the product is a colourless solution that can crystallize to get a white salt of either sodium chloride or ammonium chloride.
These are clearly very similar reactions. The full equations are:
NaOH(aq) + HCl(aq) → NaCl(aq) + H2O(l)
NH3(aq) + HCl(aq) → NH4Cl(aq)
In the sodium hydroxide case, hydrogen ions from the acid are reacting with hydroxide ions from the sodium hydroxide and it is in line with the Arrhenius theory. However, in the ammonia case, there are no hydroxide ions.
2. Some of the compounds do not contain hydrogen ions (H+) but still show the acidic property in aqueous medium Examples: FeCl3, CuSO4, and CO2.
FeCl3 + 3H2O ⇄ Fe(OH)3 + 3HCl
In the same way, some of the compounds do not contain hydroxide ions (OH−) but still show basic property Ex. Na2CO3, NH3.
Na2CO3 + 2H2O ⇄ 2NaOH + H2O + CO2
3. The Arrhenius acid-base concept is applicable in the aqueous state but not in the gaseous state. However, this same reaction also happens between ammonia gas and hydrogen chloride gas.
NH3(g) + HCl(g) → NH4Cl(s)
The Arrhenius theory is not applicable to the above reaction as the reaction carried out in the gaseous phase gives the same product as that of the aqueous phase.
4. Arrhenius’s theory says that H+ is available freely in aqueous solution, but essentially H+ is always hydrated to form hydronium (H3O+) ion.
H+ + H2O ⇄ H3O+(aq)
Make sure you also check our other amazing Article on : Limit Test for Arsenic IP