Principle of Limit Test for Iron IP
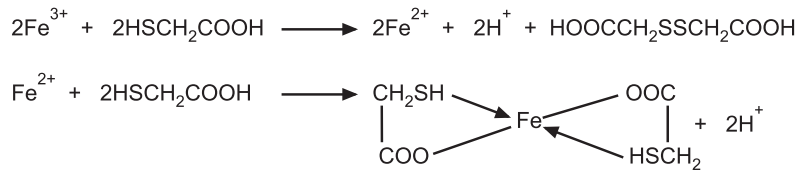
The limit test for iron is based on the formation of pale pink to deep reddish purple colour by the reaction of iron with thioglycolic acid in the presence of citric acid in a solution made alkaline with ammonia solution. The colour is due to the formation of the coordination compound, ferrous thioglycolate Fe(HSCH2COO)2. The original state of oxidation of the iron is immaterial since thioglycolic acid also reduces ferric iron to the ferrous state.
The colour produced is compared with the standard colour containing a definite quantity of iron by viewing transversely through the Nessler cylinders. Citric acid is added in the official process to prevent the precipitation of iron by ammonia. Iron gets precipitated in the form of Fe(OH)2 and Fe(OH)3 depending upon the oxidation state of the iron in the presence of ammonia. Citric acid makes a soluble complex with iron and ammonia will not be able to precipitate iron and it will just provide an alkaline medium.
Reaction:
Preparation of Reagents/Solutions:
Iron standard solution (20 ppm Fe): Dilute 1 volume of a 0.1726% w/v solution of ferric ammonium sulphate in 0.05 M sulphuric acid to 10 volumes with distilled water. It contains iron in the ferric state.
Procedure of Limit Test for Iron IP
Method (I.P. 2007):
Dissolve the specified quantity of the substance under examination in water, or prepare a solution as directed in the monograph, and transfer it to a Nessler cylinder. Add 2 ml of a 20% w/v solution of iron-free citric acid and 0.1 ml of thioglycolic acid, mix, make alkaline with iron-free ammonia solution, dilute to 50 ml with water and allow to stand for 5 minutes. Any colour produced is not more intense than that obtained by treating in the same manner 2.0 ml of iron standard solution (20 ppm Fe) in place of the solution under examination.
Example: Perform a limit test for iron in sodium chloride IP.
| Sr. No. | Test Sample | Standard |
| 1 | Place 1 g of sodium chloride and dissolve in 40 mL of distilled water in a Nessler cylinder. | Place 2.0 ml of iron standard solution (20 ppm Fe) in a Nessler cylinder. |
| 2 | Add 2 mL of 20% w/v solution of iron-free citric acid. | Add 2 mL of 20% w/v solution of iron-free citric acid. |
| 3 | Add 0.1 mL of thioglycolic acid. | Add 0.1 mL of thioglycolic acid. |
| 4 | Add iron-free NH3 to make it alkaline. | Add iron-free NH3 to make it alkaline. |
| 5 | Dilute with distilled water up to 50 mL. | Dilute with distilled water up to 50 mL. |
| 6 | Stir with a glass rod and allow to stand for 5 min. | Stir with a glass rod and allow to stand for 5 min. |
Interpretation: Compare the intensity of colour produced. For passing the test, the intensity of colour produced in the test sample should not be more than that of the standard.
Make sure you also check our other amazing Article on : Limit Test For Sulphate IP
