There are two methods for limit test for lead which are as follows:
(A) METHOD (I.P. 2007)
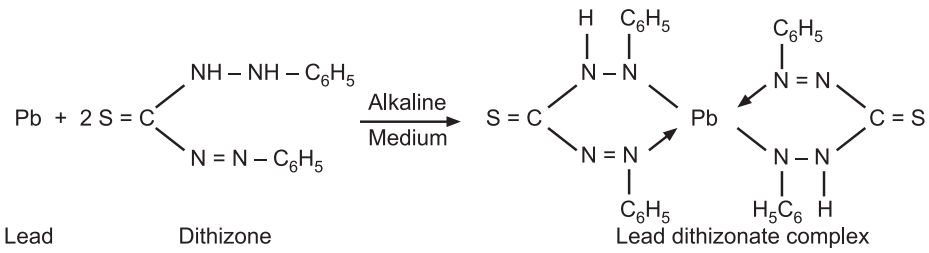
Principle: The limit test for lead is based on the reaction between lead and diphenylthiocarbazone (dithizone) in an alkaline medium to form lead–dithizonate complex. The lead present as an impurity in the substance is separated by extracting an alkaline solution with a dithizone extraction solution. The interference by other metal ions is eliminated by adjusting the optimum pH for the extraction by using reagents like ammonium citrate, potassium cyanide and hydroxylamine hydrochloride.
The original colour of dithizone in chloroform is green while the lead-dithizonate complex is violet in colour. The intensity of the violet colour of the complex depending upon the quantity of lead present in the solution is compared with that of the standard colour produced by treating a standard solution containing a definite amount of lead in a similar manner.
Reaction:
Preparation of Reagents/Solutions:
1. Standard lead solution (1 ppm Pb): Dissolve 0.400 g of lead nitrate in water containing 2.0 mL of dilute nitric acid and add sufficient water to produce 250.0 mL. This gives a standard lead solution (1% Pb). Standard lead solution (1 ppm Pb) is prepared by diluting 1 volume of standard lead solution (1% Pb) to 1000 volumes with water.
2. Dithizone extraction solution: Dissolve 30 mg of dithizone in 1000 mL of chloroform and add 5.0 mL of ethanol (95%). The solution is stored in a refrigerator. Before use, the solution is shaken with about half of its volume of 1% v/v nitric acid solution and the acid is discarded.
3. Dithizone standard solution: Dissolve 10 mg of dithizone in 1000 mL of chloroform.
Procedure:
1. Transfer the volume of the prepared sample directed in the monograph to a separator.
2. Unless otherwise directed in the monograph, add 6 ml of ammonium citrate solution and 2 ml of hydroxylamine hydrochloride solution. (For the determination of lead in iron salts, use 10 ml of ammonium citrate solution).
3. Add two drops of phenol red solution and make the solution just alkaline (red in colour) by the addition of a strong ammonia solution.
4. Cool the solution if necessary.
5. Add 2 ml of potassium cyanide solution.
6. Immediately extract the solution with several quantities, each of 5 ml, of dithizone extraction solution.
7. Drain off each extract into another separating funnel, until the dithizone extraction solution retains its green colour.
8. Shake the combined dithizone solution for 30 seconds with 30 ml of a 1% v/v solution of nitric acid and discard the chloroform layer.
9. Add to the acid solution exactly 5 ml of dithizone standard solution and shake for 30 seconds.
10. The colour of the chloroform layer is not more intense than that obtained by treating in the same manner a volume of lead standard solution (1 ppm Pb) equivalent to the amount of lead permitted in the substance under examination, in place of the solution under examination.
(B) METHOD (B.P)
Principle: In this method, the lead salts are treated with sodium sulphide which results in the formation of a black precipitate of lead sulphide. However, even a very dilute solution gives a brown precipitate with sodium sulphide solution.
Lead salts + Na2S → Sodium salt + PbS
Metals like copper and iron may interfere by producing black precipitate/brown colour by reacting with sodium sulphide. To overcome such a problem, the test is carried out in the presence of NH3 and KCN which forms a complex with cyanide and which further prevents the precipitation of copper and iron as sulphides.
Preparation of Reagents/ Solutions:
1. Test solution: It contains a definite amount of test substance.
2. Standard solution: It contains a very small amount of test sample and a specified amount of standard lead solution, i.e. lead nitrate solution.
Example: Perform a limit test for lead in ammonium chloride.
| Sr. No. | Test | Standard |
| 1 | Place 12 g of ammonium chloride in a Nessler cylinder. | Place 2 g of ammonium chloride in the Nessler cylinder. |
| 2 | Dissolve in hot distilled water cantaining 5 mL of CH3COOH. | Dissolve in hot distilled water cantaining 5 mL of CH3COOH. Add 5 mL of dilute solution of lead. |
| 3 | Make it alkaline using an NH3 solution. | Make it alkaline using an NH3 solution. |
| 4 | Add 1 mL KCN solution. | Add 1 mL KCN solution. |
| 5 | Compare the intensity. | Compare the intensity. |
| Any noticeable change in colour can be removed by adding a very dilute solution of burnt sugar | ||
| 6 | Add more distilled water up to 50 mL mark. | Add more distilled water up to 50 mL mark. |
| 7 | Add 2 drops of sodium sulphide solution. | Add 2 drops of sodium sulphide solution. |
| 8 | Stir with a glass rod and stand aside for 5 minutes. | Stir with a glass rod and stand aside for 5 minutes. |
Interpretation: Compare the intensity of colour in the two Nessler cylinders by viewing vertically downwards. For passing the test, the intensity of colour produced in the test sample should not be more than that of the standard.
Make sure you also check our other amazing Article on : Limit Test For Sulphate IP
