Principle of Limit Test for Iron
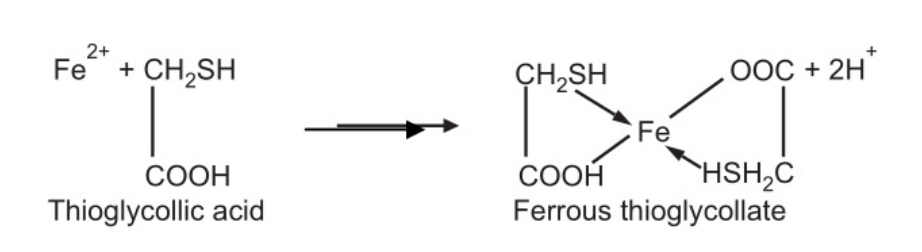
Limit Test for Iron depends upon the reaction between ferrous iron and thioglycolic acid in the presence of ammonia. A pale pink to deep reddish-purple colour is produced. Ferric iron is reduced to ferrous iron by the thioglycolic acid and the compound produced is ferrous thioglycollate. Citric acid forms a soluble complex with iron and prevents its precipitation by ammonia as ferrous hydroxide. Ferrous thioglycollate is colourless in neutral or acid solution. The colour develops only in the presence of alkali. It is stable in the absence of air but fades when exposed to air due to oxidation to the ferric compound. Therefore, the colours should be compared immediately after the time allowed for the full development of colour is over.
Apparatus Required:
- Nessler cylinders
- Glass rod
- Stand
Chemicals Required:
- Standard Iron solution Ferric ammonium sulphate (1.726 g) dissolved in 10 ml of 0.1 N H₂SO4 and sufficient water to produce 1000 ml.
- Sulphuric acid (0. 1 N): 10.0 ml.
- Iron-free citric acid solution (20% w/v): 2.0 ml.
- Thioglycolic acid: 0.1 ml.
- Iron-free ammonia solution: 20 ml.
Reaction:
Procedure for Limit Test for Iron
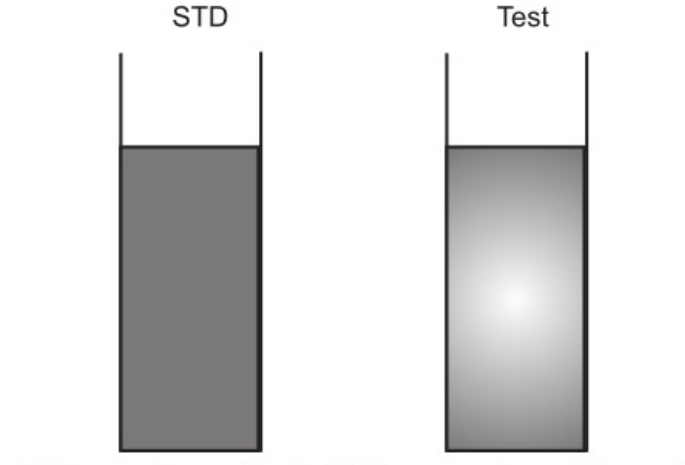
Take two 50 ml Nessler cylinders. Label one as “Test” and the other as “Standard”.
| Standard | Test |
| 1. Dilute 2 ml of standard iron solution with 20 ml of water in a Nessler cylinder. | 1. Dissolve the specified quantity of the substance in distilled water and transfer it to the Nessler cylinder. |
| 2. Add 2 ml of 20% w/v solution of iron-free citric acid and 0.1 ml of thioglycolic acid and mix. | 2. Add 2 ml of 20% w/v solution of iron-free citric acid and 0.1 ml of thioglycolic acid and mix. |
| 3. Make alkaline with iron-free ammonia solution. | 3. Make alkaline with iron-free ammonia solution. |
| 4. Dilute to 50 ml with water. | 4. Dilute to 50 ml with water. |
| 5. Observe the intensity of the purple colour developed by viewing vertically and compare it with that of the sample. | 5. Observe the intensity of the purple colour developed by viewing vertically and compare it with that of the standard. |
Table: Procedure for Limit Test for Iron
Make sure you also check our other amazing Article on : Limit Test for Sulphate
