Sun Protection: The rise in the incidence of skin cancers over the past decades is strongly related to increasingly popular outdoor activities and recreational exposure. Overexposure to sunlight is widely accepted as the underlying cause for harmful effects on the skin, eye, and immune system. Experts believe that four out of five cases of skin cancer could be prevented, as UV damage is mostly avoidable. Sun protection is essential to skin cancer prevention – about 90 percent of non-melanoma skin cancers and about 86 percent of melanomas are associated with exposure to UV radiation from the sun. Sun protection programs are urgently needed to raise awareness of the health hazards of UV radiation and to achieve lifestyle changes that will arrest the trend towards more and more skin cancers. Shade, clothing, and hats provide the best protection – applying sunscreen becomes necessary on those parts of the body that remain exposed like the face and hands. Sunscreen should never be used to prolong sun exposure. Protection against the sun may be achieved by observing the following simple steps.
Limit Time in the Mid-day Sun
The UV rays are the strongest between 10 a.m. and 4 p.m. To the extent possible, limit exposure to the sun during these hours.
Watch for the UV Index
This important resource helps in planning outdoor activities in ways that prevent overexposure to the sun’s rays. While precautions against overexposure should be taken, special care needs to be taken to adopt sun safety practices when the UV Index predicts exposure levels of moderate or above.
Use Shade Wisely
Seek shade when UV rays are the most intense. The shade structures such as trees, umbrellas, or canopies do not offer complete sun protection. Follow the shadow rule: “Watch your shadow – Short shadow, seek the shade”.
Wear Protective Clothing
A hat with a wide brim offers good sun protection to eyes, ears, face, neck, and back. Sunglasses that provide 99 to 100 percent UV-A and UV-B protection greatly reduce eye damage from sun exposure. Tightly woven, loose-fitting clothes provide additional protection from the sun.
Use Sunscreen
Apply a broad-spectrum sunscreen of SPF 15 liberally and re-apply every two hours, or after working, swimming, playing, or exercising outdoors.
Avoid Sunlamps and Tanning Parlours
Sunbeds damage the skin and unprotected eyes and are best avoided entirely.
Sunscreens
Table of Contents
Sunscreen, also known as suncream or sunblock, is a lotion, spray, gel, or other topical product that absorbs or reflects some of the sun’s ultraviolet (UV) radiation and thus helps to protect against sunburn. Skin-lightening products have sunscreen to protect lightened skin because light skin is more susceptible to sun damage than darker skin. Several sunscreens have tanning powder to help the skin to darken or tan; however, tanning powder does not protect from UV rays.
Depending on the mode of action, sunscreens can be classified into
- Physical sunscreens (those that reflect the sunlight),
- Chemical sunscreens (those that absorb the UV light).
Physical Sunscreens
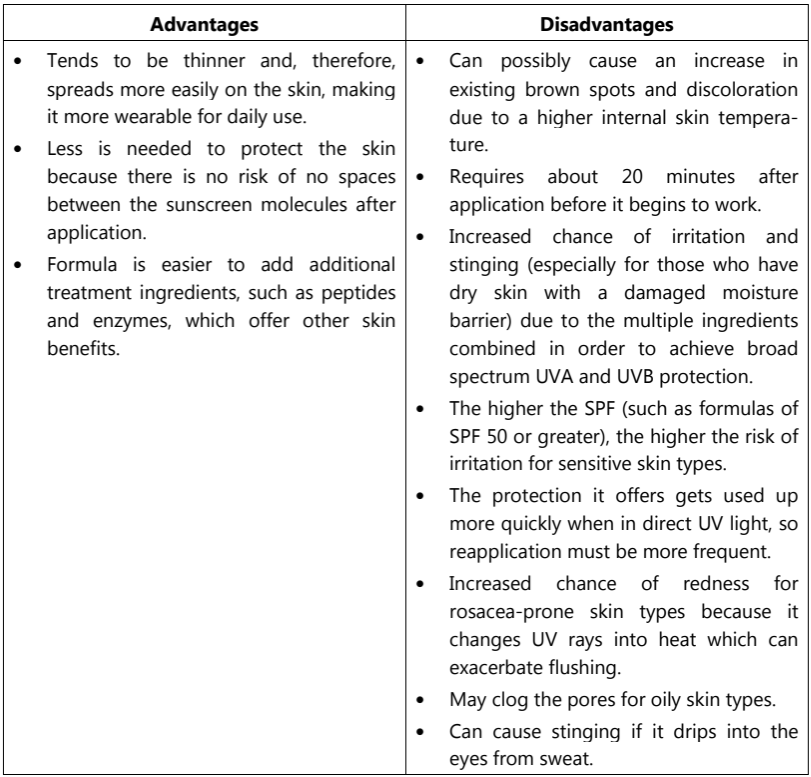
Physical sunscreens contain active mineral ingredients, such as titanium dioxide or zinc oxide, which work by sitting on top of the skin to deflect and scatter damaging UV rays away from the skin. They are often referred to as physical blockers. Physical blockers offer almost complete protection but are thick, opaque, and greasy preparations. Red petrolatum, titanium oxide, and zinc oxide are physical blockers and offer excellent protection against UVA and UVB. Physical blockers have the added benefit of being water-resistant. The table below lists the advantages and disadvantages of physical sunscreens.

Chemical Sunscreens
Chemical sunscreens contain organic (carbon-based) compounds, such as oxybenzone, octinoxate, octisalate, and avobenzone, which create a chemical reaction and work by changing UV rays into heat, then releasing that heat from the skin. They are often referred to as chemical or organic absorbers. Chemical agents commonly used as sunscreens can be broadly classified into four categories.
(i) Aminobenzoic Acid Derivatives:
- Para-aminobenzoic acid (PABA): Absorbs UVA radiation only.
- Padimate O: Extensive UVB protection.
- Methyl anthranilate: Extensive UVB protection, partial UVA protection.
(ii) Benzophenones:
- Avobenzone: Extensive UVA protection, limited UVB protection.
- Dioxybenzone and oxybenzone: Partial UVA protection, extensive UVB protection.
- Sulisonbenzone: Absorbs most UVB radiation, some UVA radiation.
(iii) Cinnamates:
- Cinoxate: Limited UVA protection extensive UVB coverage.
- Octocrylene and Octyl methoxycinnamate: Extensive UVB and limited UVA protection.
(iv) Salicylates:
- Homosalate and Octyl salicylate: UVB absorption only.
- Trolamine salicylate: Extensive UVB protection.
A combination sunscreen that combines both physical blockers and broad-spectrum chemical absorbers offers the most complete protection.
The following table lists the advantages and disadvantages of chemical sunscreens.
Besides these two categories based on the mode of action, another method to classify is based on the physical state/ chemical composition. Accordingly, the sunscreens are divided into (i) Particulate sunscreens (Organic and inorganic particulate sunscreens) and soluble (oil and water-soluble). Accordingly, other categories are described below.
1. Organic particulate Sunscreens
Organic particulates mostly absorb UV light like organic chemical compounds but contain multiple chromophores that reflect and scatter a fraction of light like inorganic particulates (e.g. Tinosorb M). Tinosorb® M is the first organic UV filter using microfine particle technology and acts as both a micro pigment and a UV absorber. This product is a white dispersion, with a pH value of 10.5-12.0. The mode of action is about 90% by absorption and 10% by scattering.
Polymeric Sunscreens
The interest in developing new sunscreens is increasing due to the harmful effects of UV radiation on the skin, such as erythema, accelerated skin aging (photoaging), and the induction of skin cancer. However, many molecular sunscreens penetrate the skin causing photoallergies, phototoxic reactions, and skin irritation. To overcome the deficiencies of sunscreen agent blends which are quickly removable upon contact with fresh or saltwater, polymeric sunscreening agents have been proposed wherein the sunscreening agent is chemically bound in the polymeric backbone of the compound, thus making the compound more skin substantive. An example of such a polymeric sunscreening agent is described in U.S. Patent No.3,529,055 which employs a copolymer containing the sunscreening agent and a hydroxylated co-monomer in a low viscosity or low molecular weight composition. Although polymeric sunscreening agents fulfill the primary function of absorbing the ultraviolet radiation which causes harmful sunburn, they are not sufficiently resistant to removal by saltwater under extended periods of exposure and do not possess good adhesion to the skin when dry. The polymeric sunscreen agents in another invention comprised of interpolymers of (a) an olefinic p-aminobenzoate devoid of hydroxy substitution; (b) N-vinylpyrrolidone; (c) a monomer selected from the group consisting of a vinyl lactam having a number average molecular weight of at least 125, an acrylate or methacrylate or any mixture thereof and optionally (d) acrylic or methacrylic acid. The interpolymeric sunscreening agent has improved UV absorption properties, resistant to crystallization on drying and to attack by saltwater for extended periods.
Others
The effectiveness of organic sunscreens is limited by phototoxicity and degradation. Both of which can be significantly reduced by encapsulation in hollow particles or covalent incorporation into the solid structure of particles. New hybrid organic/inorganic polysilsesquioxane–silica particles have been reported as sunscreens. 2-Ethylhexyl salicylate was physically encapsulated in hollow silica nanoparticles prepared by oil-in-water (O/W) microemulsion polymerization.
Sunscreening Agents
Sunscreening agents are used in some hair care products such as shampoos, conditioners, and styling agents to protect against protein degradation and color loss. Currently, benzophenone-4 and Ethylhexyl methoxycinnamate are the two sunscreens most commonly used in hair products. The common sunscreens used on skin are rarely used for hair products due to their texture and weight effects.
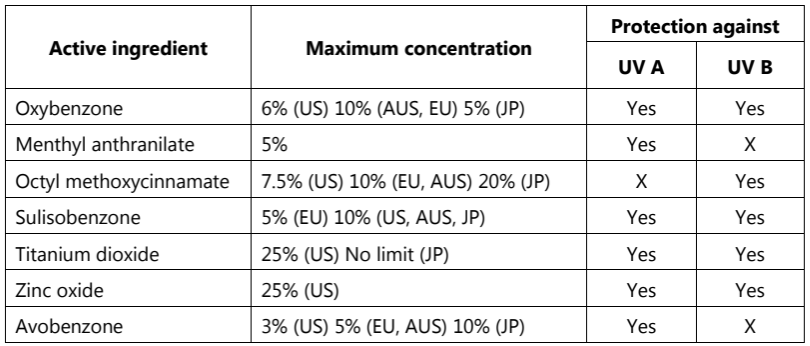
The following are the FDA allowable active ingredients in sunscreen formulations:
Table.1
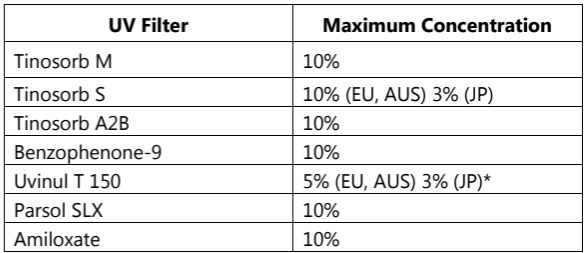
Other ingredients approved within the EU and other parts of the world, that have not been included in the current FDA Monograph include the following:
Table.2
Medical organizations such as the American Cancer Society recommend the use of sunscreens because it aids in the prevention of squamous cell carcinomas. Many sunscreens do not block UVA radiation, which does not primarily cause sunburn but can increase the rate of melanoma and photodermatitis. The use of broad-spectrum (UVA/UVB) sunscreens can address this concern. Diligent use of sunscreen can also slow or temporarily prevent the development of wrinkles and sagging skin.
Baby Sunscreens
In adults, sunburns are painful and cause tissue damage that can lead to skin cancer. But in babies, sunburns can be a medical emergency, causing dehydration, high fever, blisters, infections, chills, heatstroke and vastly increasing their lifetime skin cancer risk. Babies are not only likelier to become seriously ill from sun overexposure, but also more apt to develop sunburns: their sensitive skin contains less melanin, the pigment that gives our hair and eyes their color and offers some sun protection. In short, parents must do everything they can to keep babies safe from sunburn. The best approach is to keep infants under 6 months out of the sun and to particularly avoid exposure to the sun in the hours between 10 a.m. and 2 p.m. when ultraviolet (UV) rays are most intense. Apart from sun protection practices use of sunscreen is also recommended.
The US Food and Drug Administration and The Skin Cancer Foundation recommend using sunscreen only on children older than six months; the Foundation further recommends keeping babies out of the direct sun for the first six months. The American Academy of Pediatrics, on the other hand, suggests applying sunscreen to small areas of exposed skin in infants younger than six months old if appropriate clothing and shade are not available. Using sunscreen on newborns is quite controversial. There are key differences between infant and adult skin. First, infants’ skin is thinner, with a thinner stratum corneum, the dead outermost skin layer. It thus protects the body less effectively against outside agents; the chemicals in sunscreen might penetrate deeper, making newborns more vulnerable to contact dermatitis (skin reactions, like rashes), allergies, or inflammation. Newborn skin is also missing a functioning acid mantle, the film on the skin’s surface that protects the skin from bacteria, viruses, and transepidermal water loss (TEWL), a condition that can lead to dehydration. Lack of the acid mantle could leave babies more vulnerable to the chemicals in sunscreen.
In addition, babies also have a higher surface area to body weight ratio than older children and adults. A baby’s body surface area is about four times the body surface area to weight ratio in adults, which again leaves them exposed to greater penetration by chemicals. In adults, most sunscreen ingredients don’t get absorbed systemically (by the bloodstream) — and those that do are absorbed in insignificant amounts — but a baby’s skin may be considerably more vulnerable to this absorption; thus, it is wise to avoid any ingredients that could cause babies problems. Whether or not the ingredients in sunscreen can penetrate babies’ skin significantly, they are not meant to be ingested, and infants could very well lick them off their fingers or any other reachable body area. That is the main reason, The Skin Cancer Foundation recommends avoiding sunscreen until the baby is at least six months old. Whenever sunscreen is used on an infant, a patch test first must be performed to make sure that the baby can tolerate the product. Sunscreens that consist primarily of physical or mineral sunscreen agents, like zinc oxide and titanium dioxide, are less likely to irritate babies’ skin than chemical sunscreens. Most sunscreens made for young children are mineral/physical formulations. While the micronized/nanoparticle-sized physical sunscreens (which do not leave the classic white smears on the skin) penetrate the skin too invasively, no study has ever shown these ingredients to penetrate deeper than the stratum corneum in humans. If chemical sunscreens are preferred to skip the micronized products, sunscreens containing PABA or oxybenzone, which have been associated with skin reactions should be avoided. For Children and infants above 6 months of age, sunscreen with SPF 15 or higher may be used.
Sun Protection Factor
The harmful effects of solar radiation are caused predominantly by the ultraviolet (UV) region of the electromagnetic spectrum, which can be divided into three regions: UVA, from 320 to 400 nm; UVB, from 290 to 320 nm and UVC, from 200 to 290 nm. UVC radiation is filtered by the atmosphere before reaching the earth. UVB radiation is not completely filtered out by the ozone layer and is responsible for the damage due to sunburn. UVA radiation reaches the deeper layers of the epidermis and dermis and provokes the premature aging of the skin. Ultraviolet radiations have been implicated as a causative factor of skin cancer. Due to these facts, sunscreens substances are now incorporated into everyday products such as moisturizers, creams, lotions, shampoos, mousses, and other hair and skin preparations. The regular use of these products may help to reduce the chance of the harmful effects of ultraviolet radiation. However, a very efficient sunscreen substance must be used in the cosmetic formulation.
The efficacy of a sunscreen is usually expressed by the sun protection factor (SPF), which is defined as the UV energy required in producing a minimal erythemal dose (MED) on protected skin, divided by the UV energy required to produce a MED on unprotected skin (Eq.1).

The Minimal Erythemal Dose (MED) is defined as the lowest time interval or dosage of UV light irradiation sufficient to produce minimal, perceptible erythema on unprotected skin. The higher the SPF, the more effective is the product in preventing sunburn. Nevertheless, it is necessary to standardize methods to determine the SPF of these products.
SPF numbers on a package can range from as low as 2 to as high as 100. These numbers refer to the product’s ability to screen or block out the sun’s burning rays. For example, “SPF 15” means that 1/15th of the burning radiation reaches the skin through the recommended thickness of sunscreen. It is a common mistake to assume that the duration of effectiveness of sunscreen can be calculated simply by multiplying the SPF by the length of time it takes for an individual to suffer a burn without sunscreen because the amount of sun exposure a person receives is dependent upon more than just the length of time spent in the sun. The amount of sun exposure depends upon several factors including the length of exposure, time of day, geographic location, and weather conditions. If the skin would normally burn after 10 minutes in the sun, applying an SPF 15 sunscreen would allow one to stay in the sun without burning for approximately 150 minutes (a factor of 15 times longer). This is a rough estimate that depends on skin type, the intensity of sunlight, and the amount of sunscreen used. SPF is a measure of protection from the amount of UVB exposure and it is not meant to help you determine the duration of exposure.
For best protection, using a sunscreen of SPF 15, applying the proper amount (2 mg/cm2 of skin, or about one ounce for full body coverage), and reapplying every 2 hours is recommended.
Most individuals under-apply sunscreens, using ¼ to ½ the amount required. Using half the required amount of sunscreen only provides the square root of the SPF. So, a half application of an SPF 30 sunscreen only provides an effective SPF of 5.5 only. The SPF (Sun Protection Factor) scale is not linear. Correspondingly, SPF 15 blocks 93% of UVB rays; SPF 30 blocks 97% of UVB rays; SPF 50 blocks 98% of UVB rays. Grossly speaking SPF 30 sunscreen affords 4% more protection than SPF 15 sunscreen. In terms of photons, SPF 15 (93% protection) allows 7 out of 100 photons through; SPF 30 (97% protection) allows 3 out of 100 photons through (Figure below). Hence, while one may not be doubling the level of protection, an SPF 30 will block half the radiation that an SPF 15 would let through the skin. Most dermatologists recommend using an SPF 15 or SPF 30 sunscreen.
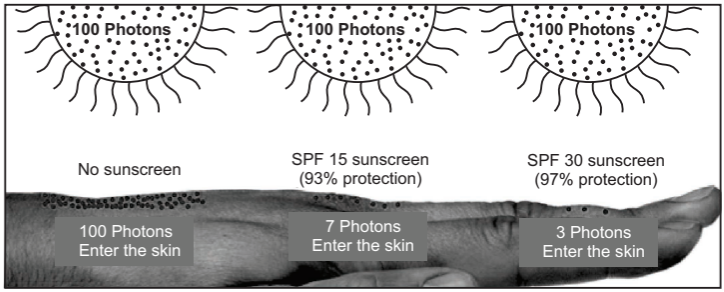
Sunscreens with really high SPFs, such as SPF 75 or SPF 100, do not offer significantly greater protection than SPF 30 and mislead that they have more protection than they do. Additionally, to have broad-spectrum protection, the UVA protection should be at least 1/3 of the UVB protection. High SPF sunscreens usually offer far greater UVB than UVA protection, thus offering a false sense of full protection.
SPF Sunscreen Testing
The photoprotection afforded by topical sunscreens against solar ultraviolet radiation exposure can be determined in vivo or in vitro, and it is ideally determined by photo testing in human volunteers. This type of determination has been used for many years and although useful and precise, is a time-consuming process, complex and expensive, particularly when information concerning the protection against long-wavelength (UVA) is required. As a consequence, much effort has been devoted to the development of in vitro techniques for assessing the photoprotection of sunscreen compounds. The in vitro methods are in general of two types: (i) Methods which involve the measurement of absorption or the transmission of UV radiation through sunscreen product films in quartz plates or biomembranes, and (ii) methods in which the absorption characteristics of the sunscreen agents are determined based on spectrophotometric analysis of dilute solutions.
All sunscreens must undergo FDA-approved SPF testing to make a UVB claim. There are three main types of SPF testing; SPF Static, SPF Water Resistant 40 Minutes, and SPF Water Resistant 80 Minutes. All sunscreen manufacturers must adhere to the same FDA-approved tests, ensuring that the SPF claims are consistent across all sunscreens, chemicals, and minerals.
SPF Static
On day one of the study, each subject receives a series of UV doses to an unprotected site on the mid-back. On the second day, the minimal erythema dose (MED) is determined as the lowest UV dose which produces mild erythema reaching the borders of the exposed site. Next, 100 mg of test sunscreen formulation is applied to a 50 cm2 area of the mid-back. After a 15 min drying period, subjects sit in a tub with moving water for 4 periods of 20 min of water immersion, separated by 20 min out of the water, totaling 80 min of accumulative water immersion. After drying for 15 min, 100 mg of the 8% homosalate standard sunscreen, which has an expected SPF of 4.47, is applied to a 50 cm2 area adjacent to the test product, then UV doses as described in the protocol are administered to the respective sunscreen protected areas. A series of UV doses are also administered to a second unprotected site. On the third day, the MED is determined for the sunscreen-protected sites and the unprotected site, and the SPF’s of each sunscreen is calculated as the ratio of the MED’s for each protected site to the MED for the unprotected site. These results are used to determine the “Very Water Resistant” SPF of the test sunscreen formulation according to the FDA Sunscreen Monograph.
Water-Resistant Sunscreens
What makes a sunscreen water-resistant? The answer to this question has two elements: the ingredient element and the legal element. But before defining these elements, it is important to understand the definition of water-resistance for sunscreens as stated in FDA’s sunscreen monograph. The water-resistance test indicates that a sunscreen product’s labeled SPF protection is retained for a certain period after immersion in water. The FDA has created the following three levels of water-resistance claims:
- Not water-resistant, hence an SPF claim only
- Water-resistant to 40 minutes
- Water-resistant to 80 minutes
The test has been described in Chapter I- FDA, Subchapter D, Part 352 – SUNSCREEN DRUG PRODUCTS FOR OVER-THE-COUNTER HUMAN USE [STAYED INDEFINITELY], Subpart D–Testing Procedures, Sec. 352.76 Determination if a product is water-resistant or very water-resistant. The general testing procedures in 352.72 shall be used as part of the following tests, except where modified in this section. An indoor freshwater pool, whirlpool, and/or jacuzzi maintained at 23 to 32o C shall be used in these testing procedures. Freshwater is clean drinking water that meets the standards in 40 CFR part 141. The pool and air temperature and the relative humidity shall be recorded.
(a) Procedure for testing the water-resistance of a sunscreen product: For sunscreen products claiming “water-resistant,” the label SPF shall be the label SPF value determined after 40 minutes of water immersion using the following procedure for the water-resistance test:
- Apply sunscreen product (followed by the waiting period after application of the sunscreen product indicated on the product labeling).
- 20 minutes moderate activity in water.
- 20 minutes rest period (do not towel test sites).
- 20 minutes moderate activity in water.
- Conclude water test (air dry test sites without toweling).
- Begin solar simulator exposure to test site areas as described in 352.73.
(b) Procedure for testing a very water-resistant sunscreen product: For sunscreen products claiming “very water-resistant,” the label SPF shall be the label SPF value determined after 80 minutes of water immersion using the following procedure for the very water resistant test:
- Apply sunscreen product (followed by the waiting period after application of the sunscreen product indicated on the product labeling).
- 20 minutes moderate activity in water.
- 20 minutes rest period (do not towel test sites).
- 20 minutes moderate activity in water.
- 20 minutes rest period (do not towel test sites).
- 20 minutes moderate activity in water.
- 20 minutes rest period (do not towel test sites).
- 20 minutes moderate activity in water.
- Conclude water test (air dry test sites without toweling).
- Begin solar simulator exposure to test site areas as described in 352.73.
The pool and air temperature and the relative humidity are recorded. After the water immersion/rest period cycle, the test sites are allowed to air-dry without toweling before exposure from the solar simulator. Sixteen to twenty-four hours post-exposure, the subjects are instructed to return to the facility for evaluation of delayed erythema (skin reddening) responses. The FDA set these time limits based on research done in 1978 that indicated that swimmers in the pool or the ocean, spend an average of 21 min in the water and go in the water an average of 3.6 times on any given day.
The Ingredient Element: To make a sunscreen water-resistant, ingredients have to be added that will allow the sunscreen formulation to adhere to skin when submerged in “swirling water”. This can be done with waxes, oils, or a type of polymer such as dimethicone. Because accepted sunscreen active ingredients are either some form of a liquid chemical compound or a fine mineral powder, they do not generally adhere to human skin. The formulator’s objective is to mix ingredients into the sunscreen that will:
- help the sunscreen active ingredients adhere to skin when immersed in water.
- not negatively affect the balance of the sunscreen formulation, causing it to separate.
- not inhibit sunscreen ingredients from achieving desired SPF levels.
- not fill and plug skin pores.
The Legal Element: The ability to claim a water-resistant capability is solely dependent on a product’s performance in a water-resistance test described above. The protocol for this test was designed by the FDA to simulate actual swimmer behavior. The SPF of a product being tested is recorded as many as three times during water-resistant testing:
- In the beginning without submersion.
- At the end of the second submersion or 40 min in the water.
- At the end of the fourth submersion or 80 min in the water.
A manufacturer can consider each of these three SPF readings and choose which one they want to use on the label of their product. For example, if a product scores an SPF 58 without the water testing and an SPF 53 after the fourth submersion (80 min). The product can be labeled as SPF 58, but then water resistance could not have been mentioned on the label. To have the “Water-Resistant (80 minutes)” added to the label, the SPF can only be as SPF 53.
The water-resistant issue that this testing protocol does not directly address is that of sweating. Generally speaking, sweating produces a 98% water liquid, and because sweat emerges from within the skin, it has the possibility of washing sunscreen away as it leaves the skin’s pores. While this could happen, its impact on overall sunscreen protection is very negligible. Skin pores range in size from 500-700 microns in diameter and vary in their distribution over the body. For example, a standard accepted pore distribution on the forehead is 175-200 pores per cm. These dimensions suggest that on the forehead, skin pores only represent 0.2% of the skin’s surface. The performance of a sunscreen that has been tested for water resistance would not be affected due to sweat from such a small portion of the overall skin. What can happen, however, is that a sweating person can wipe the sweat off of their body or face, which would also wipe off their sunscreen.
Make sure you also check our other amazing Article on : Prevention of Mouth Ulcers
