Synthesis and Reactions of Acridine: Acridine is a colorless aromatic compound having a melting point of 106-110°C. It is structurally related to anthracene with one of the central atoms replaced by nitrogen. It was first isolated from coal tar in 1870 by Carl Grabe and Heinrich Caro. Like pyridine, it is mildly basic.
Chemical Synthesis of Acridine
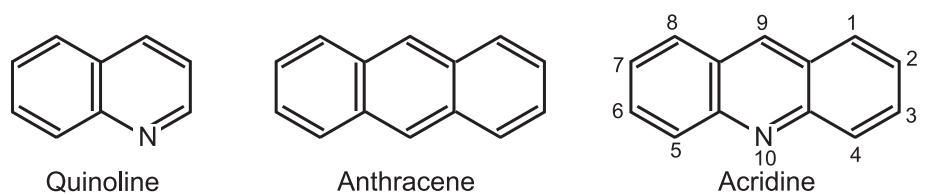
(1) Bernthsen acridine synthesis: When diphenylamine is condensed with carboxylic acids in presence of zinc chloride, it provides acridines.
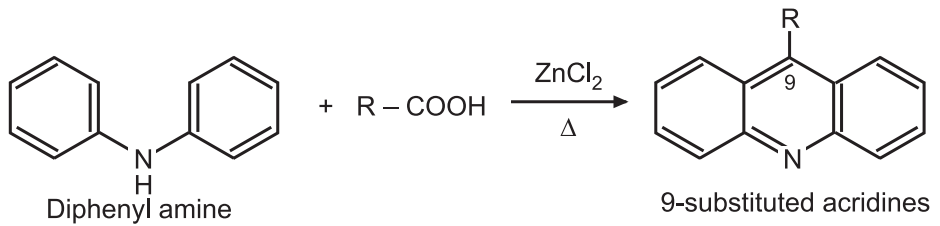
(2) From o-chlorobenzene acid (Ullmann synthesis): Aniline and o-chlorobenzene acid are condensed to form diphenylamine-2-carboxylic acid. This acid is cyclized with POCl3 to give 9-chloroacridine.
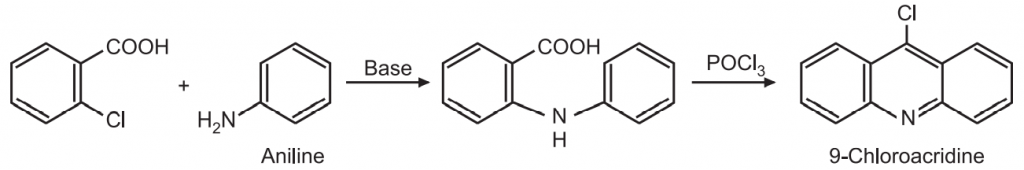
The 9-chloroacridine is first reduced by catalytic hydrogenation followed by oxidation using ferric chloride to give acridine.
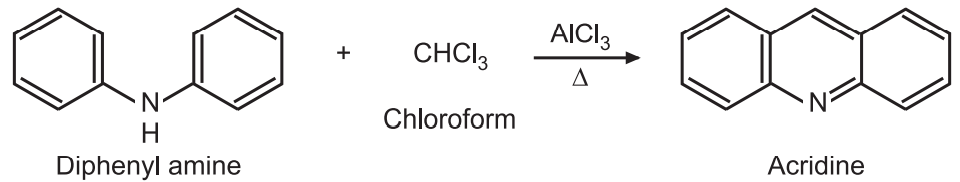
(3) Diphenylamine is condensed with chloroform in presence of AlCl3 to give acridine.
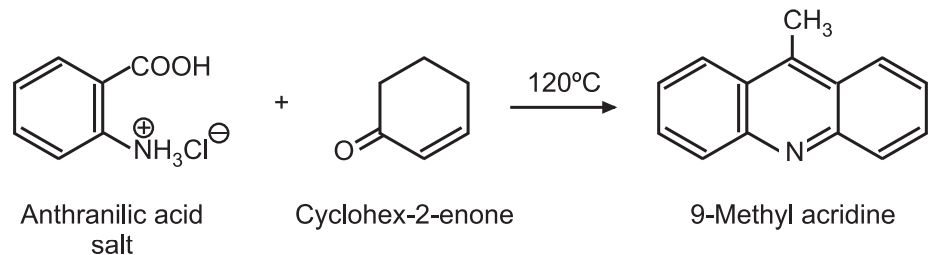
(4) Friedlander synthesis: The salt of anthranilic acid is treated with cyclohexane-2-enone at 120°C to give 9-methyl acridine.
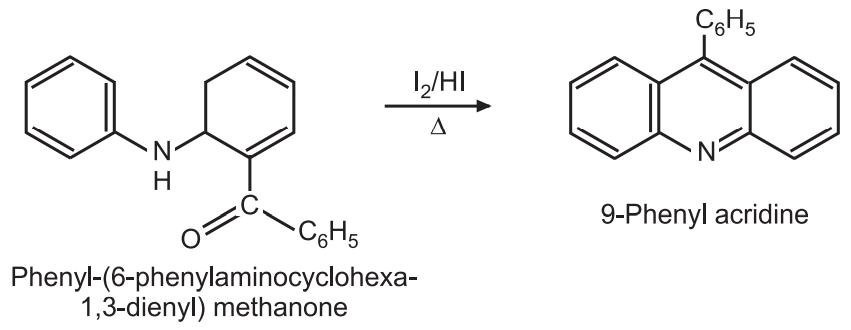
(5) From C-acylated diphenylamines: The diphenylamine is first acylated. The acylated diphenylamine is heated in the presence of I2/HI to give 9-phenylacridine.
Chemical Reactions of Acridine
Acridine is the aza derivative of anthracene. It is a weak base. The lone pair of electrons available on N-atom and aromatic nature of acridine compel it to behave chemically, similar to pyridine and quinoline.
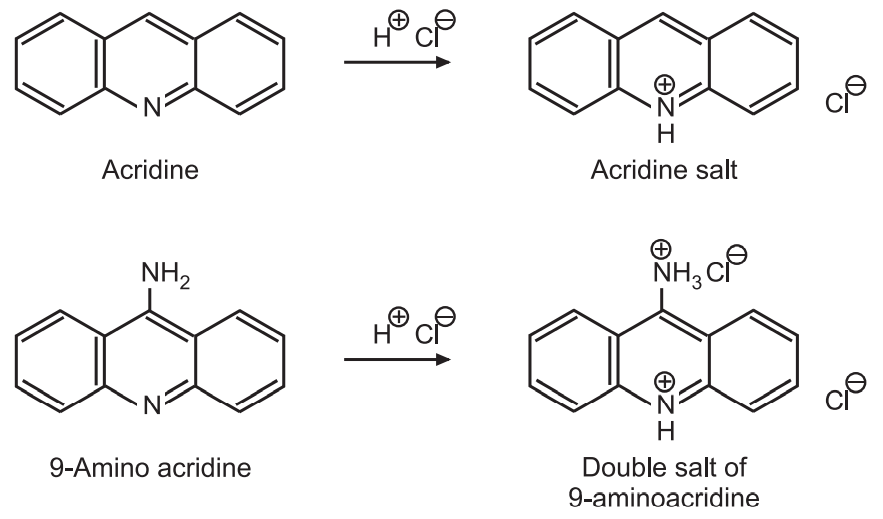
(a) N-protonation: Acridine being a weak base, forms soluble salt by protonation at N-atom using its lone pair of electrons. Amino-acridine forms a double salt where the ring N-atom is first to get protonated.
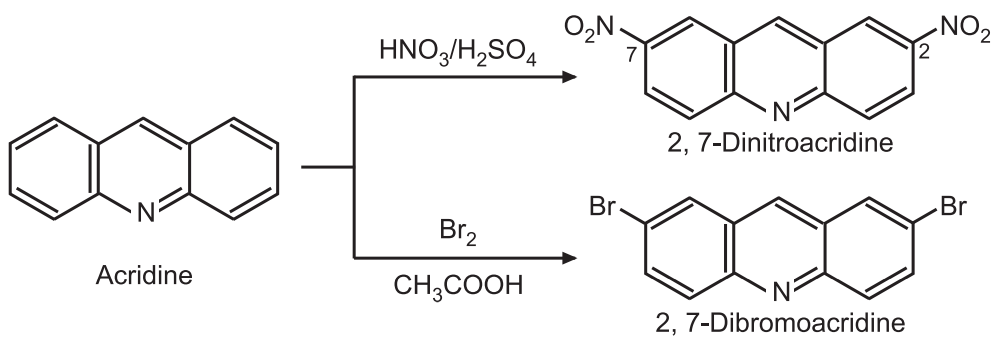
(b) Electrophilic substitution: The electrophile attacks the benzenoid ring preferably at 2- and 7-positions resulting in di-substitution. e.g.,
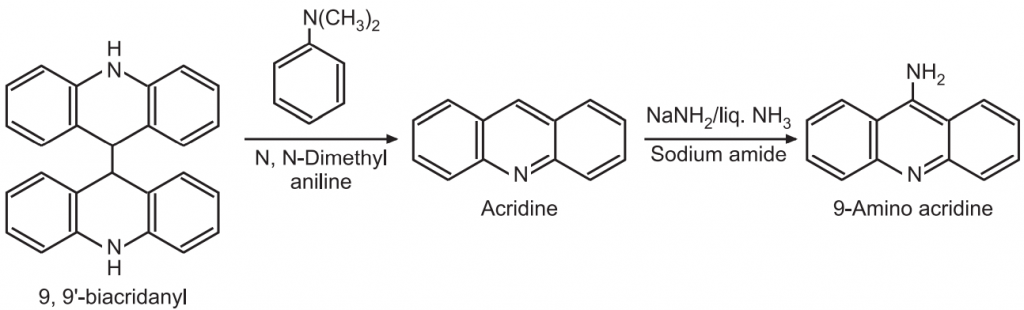
(c) Nucleophilic substitution: The quaternary salts of acridine are more reactive towards nucleophilic reagents. In acridine, the electron density at position-9 is low in comparison to 1-, 2-, 3- and 4- positions. Hence, nucleophile preferably attacks at 9- position.
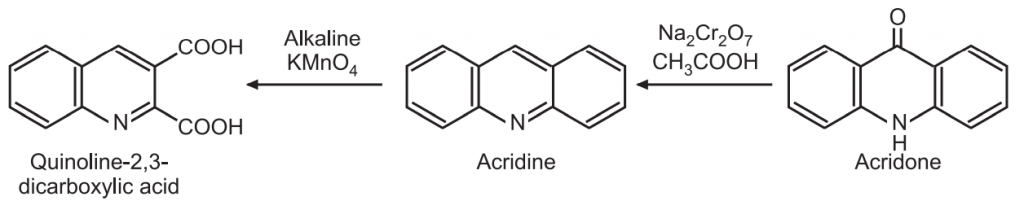
(d) Oxidation: Acridine is oxidized by dichromate in acetic acid to give acridone. The oxidative ring cleavage occurs by KMnO4 in alkaline medium forming quinoline -2, 3-dicarboxylic acid.
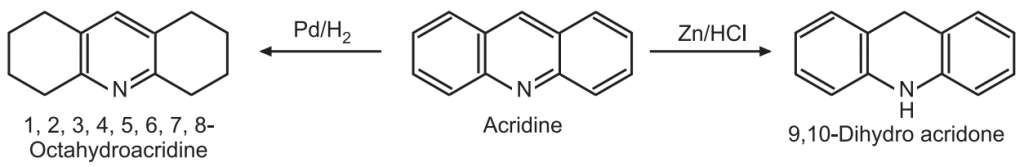
(e) Reduction: The benzene rings of acridine can be selectively reduced by catalytic hydrogenation while the pyridine ring can be selectively reduced by Zn/HCl to give 9, 10-dihydroacridine.
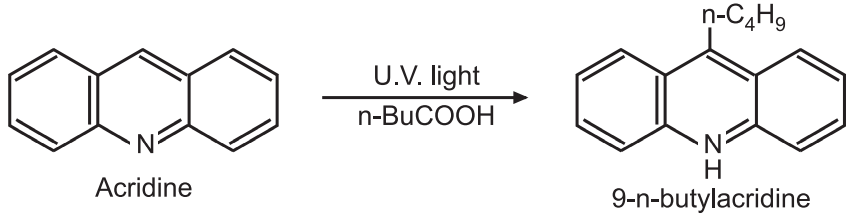
(f) Reductive alkylation: Acridine reacts with n-pentanoic acid in the presence of U. V. light to give 9-n-butylacridine.
Applications in Drug Synthesis
Many drugs in the market have acridine as a core skeleton. These drugs include bucricaine (anesthetic), quinacrine (or mepacrine: antimalarial), 9-ammoacridine (disinfectant), proflavin (antibacterial), nitracrine (anticancer), acriflavine (antiseptic), etc.
Make sure you also check our other amazing Article on : Synthesis and Reactions of Pyridine
