To provide satisfactory conditions for the proper processing of parenterals, it is necessary to monitor the area with suitable environmental control tests. These methods can be divided into general methods, air sampling methods, and surface sampling methods.
General Methods
General methods are used to validate HEPA filters, detect particulate contamination and monitor the environment. These methods include filter efficiency tests, induction leak tests, particulate contamination control tests, air pressure tests, air flow tests, noise level tests, lighting tests, and temperature and humidity tests.
Air Sampling Methods
Air sampling methods include:
(i) Electronic air particle counters: These counters are especially useful in determining the number of particle/microbes counts per cubic feet to classify the cleanliness of a particular room or area. Electronic counters count all particles/ microbes but cannot differentiate between viable and non-viable microbes.
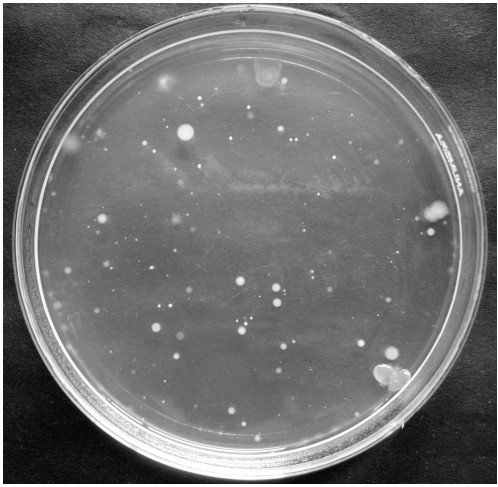
(ii) Settle plates: A nutrient agar or other suitable medium is exposed (Fig.1) to the atmosphere in the sampling area for a predetermined period (20 to 60 minutes). Plates are incubated at 30 to 35 oC for 48 hours and colonies are counted. This is a simple and inexpensive method commonly used for the detection of microbial load in clean rooms. The major disadvantage of this method is that only those microorganisms that adhere to the surface of large particles are counted. Different types of microbial growth by the settle plate method are shown in Fig.2.
(iii) Slit-air sampler: The slit-air sampler is one of the most widely used monitoring methods for manufacturing parenterals and quality control environments. This is a device that collects viable airborne microbial and particulate contamination. Trypticase soy agar or any other suitable medium is placed on a circular plate in the slit air device and the cover containing the slit is secured above the agar plate. The speed of the plate rotation and the volume of air sampled can be adjusted to record the desired rate and the degree of contamination of the air environment.
(iv) Liquid impinger: The air sample may be drawn into a measured volume of nutrient broth in an impinger. Microorganisms in the broth then may be collected by membrane filtration, incubated, and counted.
(v) Centrifugal air sampler (Biotest): Airborne microorganisms approximately 16 inches above the sterile drum housing are drawn toward the impeller blades. By applying centrifugal forces (approx. 4000 to 4200 r.p.m.), the microbial particles are impacted at a high velocity onto the agar surface of the agar strip wound around the impeller blades. After incubation, colonies on the strips are counted and recorded (CFU/unit volume of air).
Surface Sampling Methods
(i) Rodac plates: Samples of the level of microorganisms on the surface can be determined by specially built convex surface Petri plates. With the Rodac plates, it is possible to roll the raised agar surface over flat or irregular surfaces to be tested. Surface contamination can be quantified by counting the colonies after incubation at 30 to 35 oC for 48 hours.
(ii) Swab-rinse test: This is a simple surface sample method employing sterile cotton swab tips to sample locations. The swabs are then placed into tubes of culture media or sterile water (microbial counting) and a sample of the water is placed on a solid agar plate.
The purpose of environmental control of microbial contamination is to minimize the potential for product contamination. The lesser the potential for contamination, the greater the assurance that the product is sterile. The sterility test can be used primarily as a confirmation of the sterility already built into the product.
Make sure you also check our other amazing Article on : Sterility Test in Microbiology