An auxiliary substance which helps in the usual detection of the completion of the titration or equivalence point or end point is termed an indicator, i.e., substances which undergo some easily detachable changes at the equivalence point are used as indicators.
They possess one colour in the presence of an excess of the substance to be estimated and another colour in the presence of an excess of the standard solution of the reagent used and thus these substances indicate the exact end point.
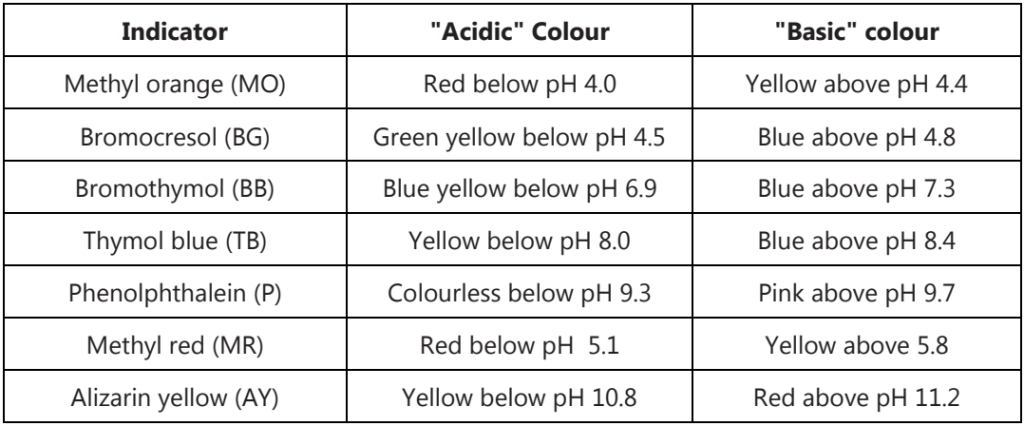
In acid-base titrations, organic substances (weak acids or weak bases) are generally used as indicators. They change their colour within a certain pH range.
Characteristics of a Good Indicator
1. Colour change must be easily detected, i.e., it must be sensitive.
2. Colour change must be rapid and sharp.
3. The indicator molecule must not react with the substance being titrated.
4. The indicator should have a pKin that is within one pH unit of the expected pH at the equivalence point of the titration.
5. The pH range over which the colour change takes place must be such as to indicate when the reaction is complete.
Choosing an indicator for an acid-base titration
1. For titrations of strong acids and strong bases (and vice versa), any indicator with a pKin between 4 and 10 should be used.
2. For the titration of a weak acid, the pH at the equivalence point is > 7, and an indicator such as phenolphthalein or thymol blue, with pKin > 7, should be used.
3. For the titration of a weak base, where the pH at the equivalence point is < 7, an indicator such as methyl red or bromcresol blue, with pKin < 7, should be used.
Basic – if the reaction involves a strong base and a weak acid.
Neutral – If the reaction involves a strong acid and a strong base.
Acidic – If the reaction involves a strong acid and weak base.
Indicators cannot give a particular numerical value for pH. They can only give a range for the pH of the solution.
Make sure you also check our other amazing Article on : Lewis Theory of Acids and Bases
