Molecular Formula: C10H13NO4
Molecular Weight: 211.21
Material Required: Methyldopa 0.2 g, anhydrous formic acid: 15 mL, glacial acetic acid: 30 mL, dioxane: 30 mL, 0.1 N perchloric acid, and crystal violet solution.
Procedure: Weigh accurately about 0.2 g of methyldopa and dissolve in a mixture of 15 mL of anhydrous formic acid, 30 mL of glacial acetic acid, and 30 mL of dioxane. Add 0.1 mL of crystal violet solution and titrate with 0.1 N perchloric acid. Perform a blank determination and make any necessary corrections.
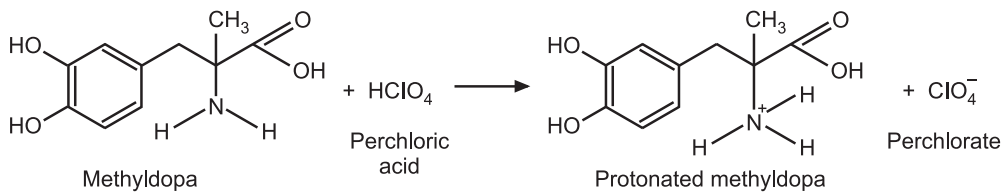
Reaction:
Factor calculation:
1 mole of perchloric acid ≅ 1 mole of methyldopa
1000 mL of 1N HClO4 ≅ 211.21 g of C10H13NO4
1 mL of 0.1N HClO4 ≅ 0.021121 g of C10H13NO4
Make sure you also check our other amazing Article on : Indicators for Non Aqueous Titrations