The polymers are classified into various types based on different categories. They are:
Classification of Polymer Based on Source
Table of Contents
1. Natural polymers: These are derived from natural sources and can be polysaccharides and proteins in chemical nature. For example: Albumin, Cellulose, Starch, Rubber, Wool.
2. Semi-synthetic polymers: These types of polymers are derived from naturally occurring polymers using chemical modifications. E.g. Vulcanized rubber, Guncotton, Cellulose diacetate, HPMC, etc.
- Vulcanized rubber is used in making tires as the process of vulcanization increases the mechanical strength of natural rubber.
- Guncotton which is a cellulose nitrate is used in making explosives. Cellulose on acetylation with acetic anhydride in the presence of sulfuric acid forms cellulose diacetate which is used in the production of treads and materials like films, glasses, etc.
3. Synthetic polymers: Synthetic polymers are of artificial origin which consist of fibers. This is the polymer, which was prepared by Laboratory is known as Synthetic Polymer. For example: Buna-S, Buna-R, Nylon, Polythene, Polyester.
Classification of Polymer Based on Structure
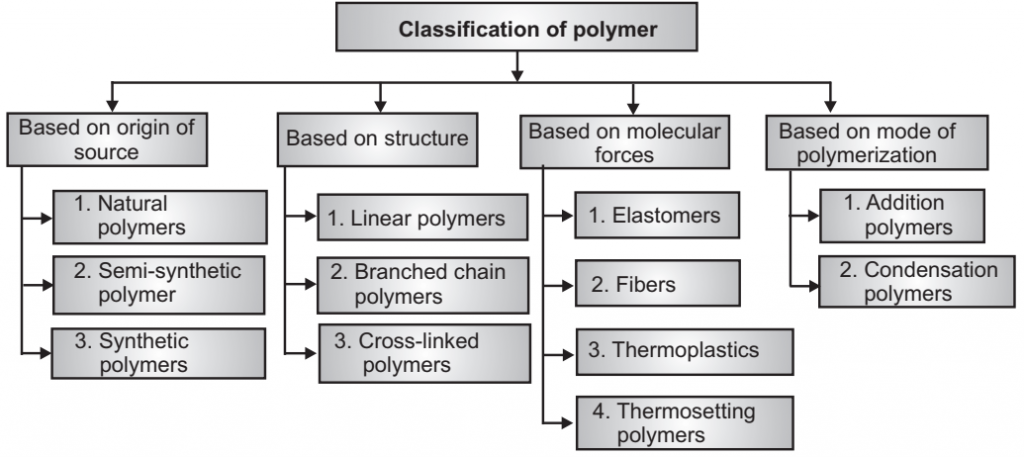
1. Linear polymers: The smallest repeating unit arranged in a straight-line path is known as Linear polymer. For example: PVC.
2. Branched-chain polymers: Contain linear chains having some branches. For example: low-density polymer, Polyethylene, HPLD polyethylene.
3. Cross-linked chain polymers: In this type, all molecules are chemically bonded together, forming a three-dimensional network. The bonding is usually covalent but other types such as; ionic bonds are also possible. Cross-linked polymers are produced from linear and branched polymers or directly from chemical precursors. E.g. Natural rubber, polyacrylamide gels, epoxies, alkyd resins, etc.
Classification of Polymer Based on Polymerization
1. Additional polymers: Additional polymers are formed by the repeated addition of monomer molecules possessing double or triple bonds.
n(CH2 = CH2) − (CH2 − CH2)−
Ethylene polyethylene, one form of the polymer is converted into another form of a polymer by loss of atoms and ions from a molecule.
2. Condensation polymers: Condensation polymers are formed by repeated condensation reactions between two different bi-functional or tri-functional monomeric units. E.g. terylene (dacron), nylon 6, 6, One polymer can be converted into another form of polymer without loss of atoms and ions from a molecule.
Classification of Polymer Based on Molecular Force
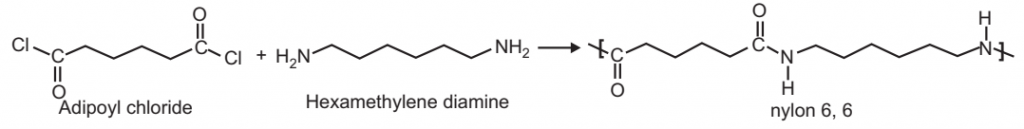
1. Nylon: Nylon is used as a general name for all synthetic fiber-forming polyamides, having a protein-like structure. These are the condensation polymers of diamine and dibasic acids. A number is usually suffixed with the Nylon which refers to the number of carbon atoms present in the diamine and the dibasic acids respectively.
For example: Nylon 6, 6. Nylon-6, 6 is obtained by the polymerization of adipic acid with hex methylene diamine.
2. Thermoplastic polymers: These are linear or slightly branched long-chain polymers, which can be softened on heating and reversibly hardened on cooling repeatedly. Their hardness is a temporary property and varies with temperature.
The polymer under heating can convert from one state to another state and after cooling, it can again convert to its original state.
For example: polyvinyl chloride.
Make sure you also check our other amazing Article on : Advantages of Polymer
