Mechanism of Drug Release from Polymer: Three primary mechanisms for drug release, namely:
- Diffusion
- Degradation
- Water penetration (Swelling)
Drug Release from Polymer by Diffusion
Rate limiting step is a diffusion of drug through inert water-insoluble membrane barrier.
There are two types:
- Reservoir
- Matrix
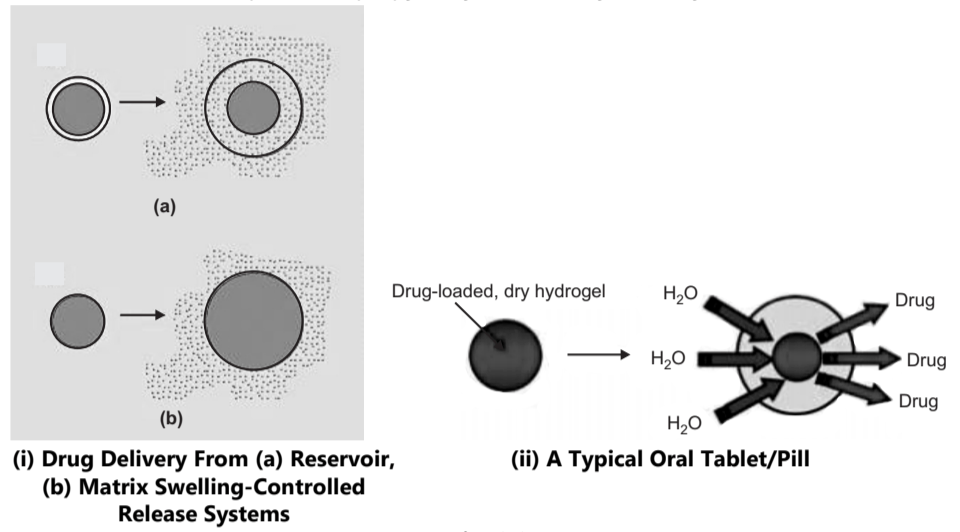
1. Reservoir Diffusion System:
In membrane-controlled reservoir devices, the drug is contained in a core, which is surrounded by a polymer membrane, and it is released by diffusion through this rate-controlling membrane.
For example, Poly (N-vinyl pyrrolidone), Poly (ethylene-co-vinyl acetate).
Drug delivery from typical reservoir devices: (i) Implantable or oral systems, and (ii) Transdermal systems.
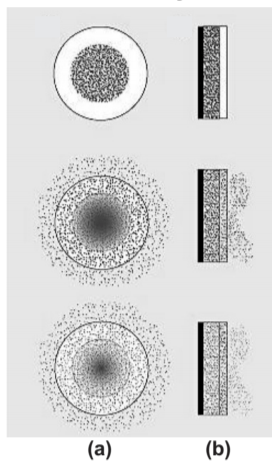
2. Matrix Diffusion System:
In these devices, the drug is released either by passing through the pores or between polymer chains, and these are the processes that control the release rate.
For example, polyethylene, polyvinyl acetate.
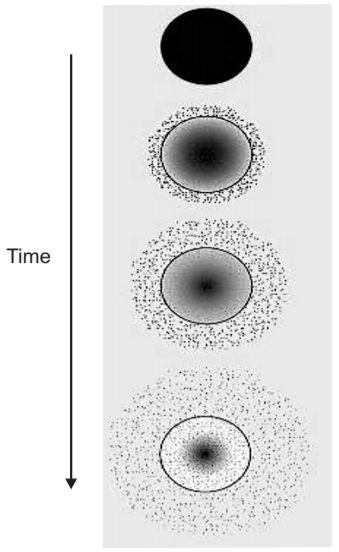
Drug Release from Polymer by Degradation
Bio-degradation is the chemical changes that alter the molecular weight or solubility of the polymers. Bio erosion may refer to a physical process that results in the weight loss of a polymer device. The possibility for a polymer to degrade and to have its degradation by-products assimilated or excreted by the living system is designated as Bioresorbable. The erosion of polymers takes place by two methods:
- Chemical erosion
- Physical erosion
1. Chemical Erosion: Bio erosions through chemical mechanisms are explained below: Mechanism-I: It describes the degradation of water-soluble macromolecules that are cross-linked to form a three-dimensional network. Degradation in these systems can occur by:
- Type (1A): Degradation occurs at crosslinks to form soluble backbone polymeric chains. It provides high molecular weight, water-soluble fragments.
- Type (1B): Degradation occurs to form water-soluble fragments. Such type provides low molecular weight, water-soluble oligomers, and monomers.
Mechanism-II: Describes the dissolution of water-insoluble macromolecules with side groups that are converted to water-insoluble polymers as a result of ionization, protonation, or hydrolysis of the groups.
Materials showing this type of erosion include;
- Cellulose acetate derivatives,
- Co-polymers of maleic anhydride.
Mechanism-III: Describes the degradation of insoluble polymers with liable bonds. It forms low molecular weight, water-soluble molecules.
Polymers undergoing this type of erosion include;
- Poly (lactic acids)
- Poly (glycolic acid) and their co-polymers, etc.
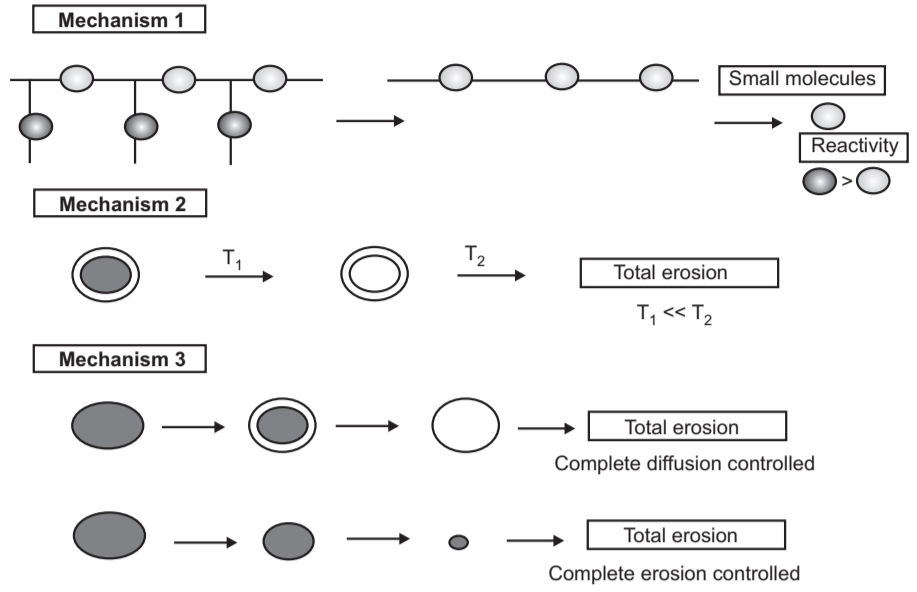
2. Physical Erosion: The physical erosion mechanisms can be characterized as heterogeneous or homogeneous.
- Most polymers undergo homogenous erosion that means the hydrolysis occurs at an even rate throughout the polymeric matrix.
- In homogenous erosion, there is a loss of integrity of the matrix or polymer.
- In heterogeneous erosion, also called surface erosion, the matrix degrades and the drug is released from the surface.
- Highly crystalline polymers tend to undergo heterogeneous erosion.
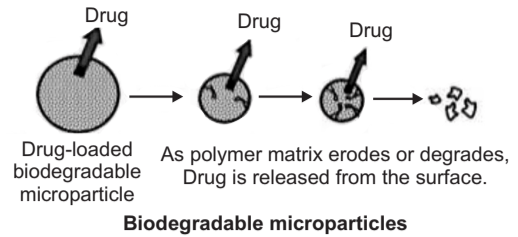
Drug Release from Polymer by Water Penetration (Swelling)
These types of systems are initially dry and when placed in the body, absorb water or other fluid and it swells. In swelling controlled release systems, an active agent is homogeneously dispersed in a glassy polymer, because glassy polymers are essentially impermeable, the active agent is immobilized in the matrix and the diffusion through the solid polymer phase takes place.
When monolithic devices are placed in an aqueous environment, water begins to penetrate the matrix and swelling takes place. As a consequence of the swelling process, chain relaxation takes place and the incorporated active agent begins to diffuse from the swollen layer.
In linear amorphous polymers, dissolution follows the swelling process, but crosslinked polymers or those containing significant chain entanglements or partial crystallinity will remain insoluble but will be mechanically weak.
Swelling increases aqueous solvent content within the formulation as well as the polymer mesh size, enabling the drug to diffuse through the swollen network into the external environment. For example: (N-isopropyl-acrylamide), Ethylene-vinyl alcohol.
Make sure you also check our other amazing Article on : Classification of Polymer