Oppenauer Oxidation: This reaction is named after Rupert Viktor Oppenauer. This method is used for selectively oxidizing secondary alcohols to ketones, using aluminum isopropoxide in excess acetone. The reaction is the opposite of Meerwein-Ponndorf-Verley reduction.
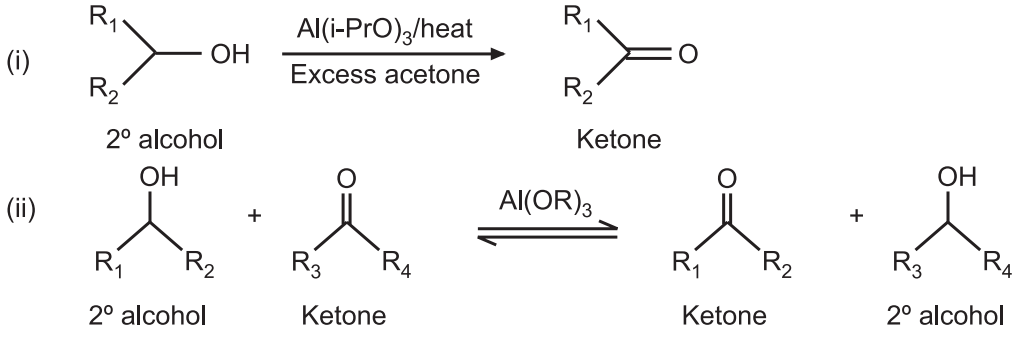
Oppenauer oxidation is an important reaction used in the synthesis of alkaloids, hormones, steroids, and terpenes. The low reactivity of aluminum alkoxide used in this reaction can be improved by tert-Bu-CHO and excess of acetone as the powerful hydride acceptor. Excess of acetone shifts the equilibrium toward the product side.
In Oppenauer oxidation, the aluminum-catalyzed hydride shift occurs from the α-carbon of an alcohol component to the carbonyl carbon of a second component.
Reaction Mechanism:
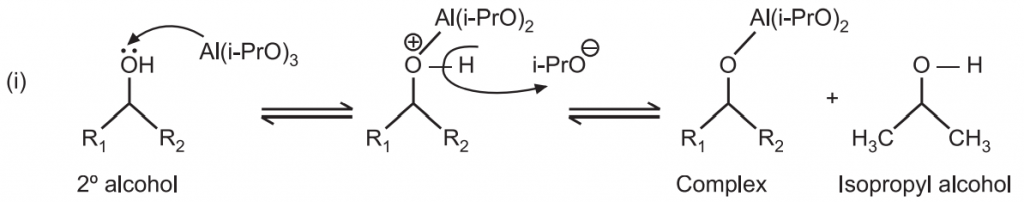
In the first step, alcohol coordinates with aluminum isopropoxide to form a complex and isopropyl alcohol. The reaction begins with the alcohol (starting material) replacing one of the isopropoxide groups on the aluminum to generate isopropyl alcohol.
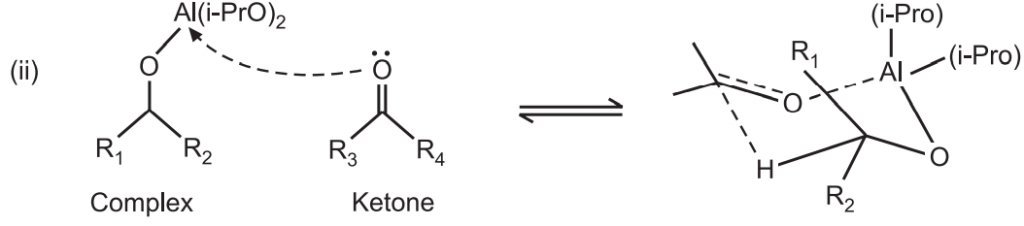
The complex reacts with a ketone (acetone) to form a six-membered transition complex.
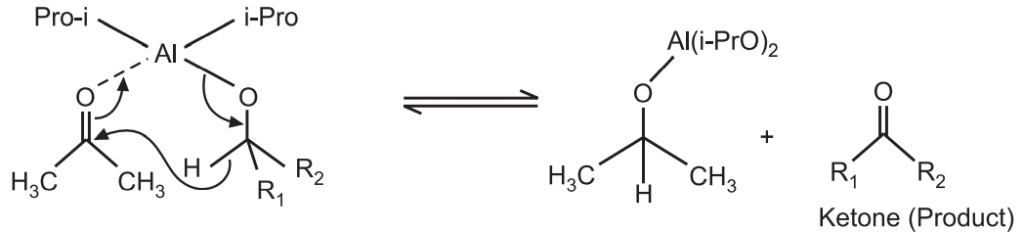
The α-carbon of the alcohol (starting material) is converted to the carbonyl carbon (product) from the aluminum catalyzed hydride shift. The desired ketone is formed after the hydride transfer.
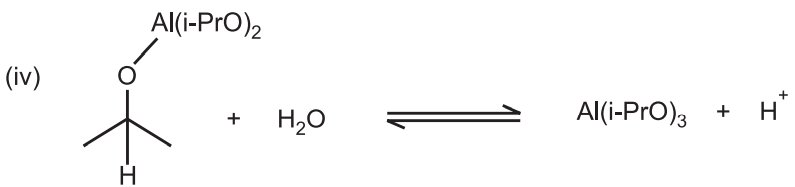
The last step thus results in the formation of the final ketone product and regeneration of the aluminum isopropoxide catalyst.
Woodward Modification: In the Woodward modification, potassium tert-butoxide is used in place of aluminum alkoxide. He used potassium tert-butoxide and benzophenone for the oxidation of quinine to a quinone.
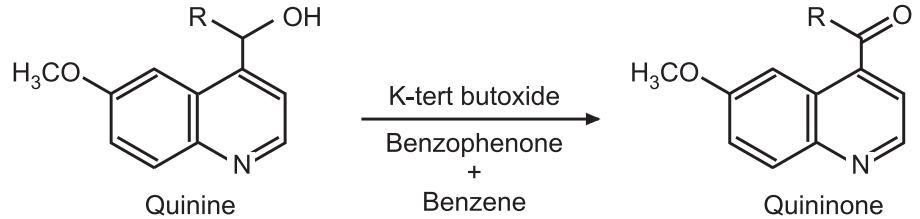
Applications in Drug synthesis:
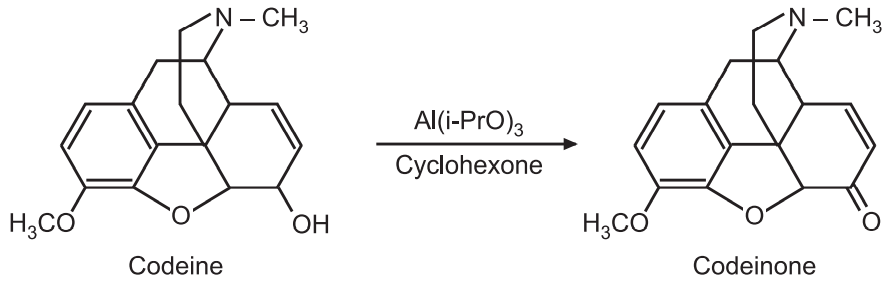
(i) The analgesic drug codeine is converted to codeinone by Oppenaure oxidation.
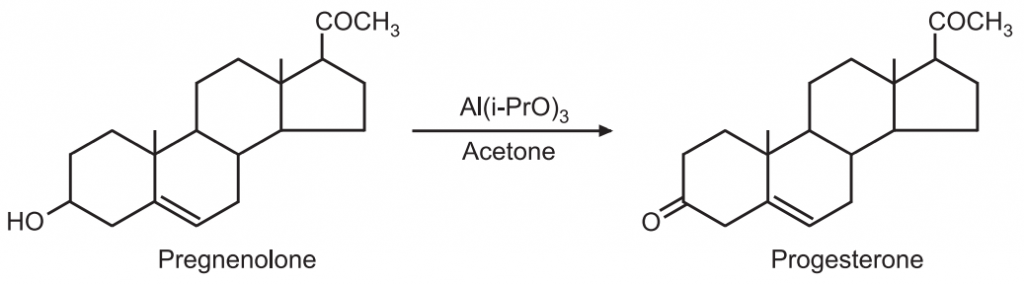
(ii) The female sex hormone progesterone is prepared by the Oppenauer oxidation of pregnenolone.
Make sure you also check our other amazing Article on : Birch Reduction
