Reactions of Chiral Molecules: Chiral molecules react with the reagents in a variety of ways and accordingly, reactions are classified as follows:
- Reactions where bonds with the chiral center are not broken.
- Reactions leading to generation of chiral center.
- Reactions of chiral compounds with optically active reagents.
- Reactions where bonds with the chiral center are broken.
1. Reactions where bonds with the chiral center are not broken:
Table of Contents
These reactions can be used to relate the configuration of one compound to that of another. Configuration is retained when the reaction does not involve the breaking of a bond to a chiral center.
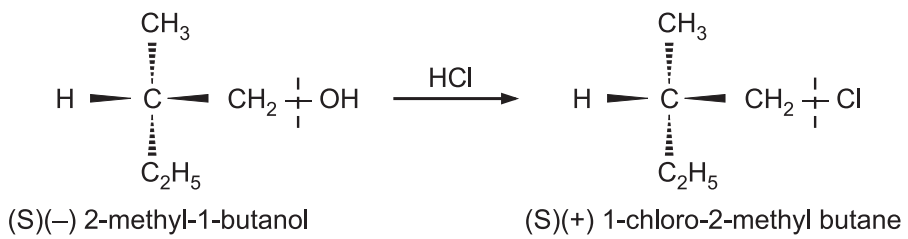
Here is the bond to the chiral center is not broken ‘S’ configuration is retained, because ‘–CH2–Cl’ occupies the same relative position as that was occupied by –CH2OH in the reactant. This retention of configuration can be utilized to determine the configurational relationship between two optically active compounds by converting them into each other by reactions that do not involve the breaking of a bond to a chiral center. Only relative configuration can be assigned than absolute configuration.
Such reactions are used to get specific rotations of optically pure compounds. e.g. 2-methyl-1-butanol from fusel oil has a specific rotation of –5.90° and is optically pure. Upon treatment with hydrogen chloride, 1-chloro 2-methyl butane has a specific rotation of +1.67°. So if a sample has rotation equal to this value, the compound is said to be pure. If rotation is about + 0.8º, the compound is said to be only 50% optically pure.
2. Reactions leading to generation of chiral center:
Generation of first chiral center in a compound usually yields equal amounts of enantiomers (Racemic mixture) but reactions that form second/new chiral center yield unequal amounts of diastereomers depending on the side of attack.

Retention of configuration(s) occurs as there is no bond breaking to the chiral center. For the new chiral center, depending on side of attack from the same or opposite side, diastereomers are formed but in unequal amounts. This is because the intermediate 3-chloro-2-butyl radical contains a chiral center and it lacks symmetry. So two faces of the molecule for attack are not equal to each other. Here S isomer would yield the SS and meso compound in the ratio of 29:71.
In some reactions, both configurations may not be generated but probability exists. Similarly, the R isomer would yield RR and meso compound in the ratio of 29:71. If the reactant is optically inactive, it yields optically inactive products.
3. Reactions of chiral compounds with optically active reagents:
Such reactions are commonly used in the resolution or separation of a racemic mixture/modification into individual enantiomers. Because enantiomers have similar physical properties (except optical rotation) they are not separated by usual methods of fractional distillation or crystallization.
So to obtain pure enantiomers from racemic modification, use of optically active reagents is made. Such optically active reagent is easily obtained from natural sources or generated from naturally available reagents.
Common reactions are reactions of organic acids and bases to form salts.
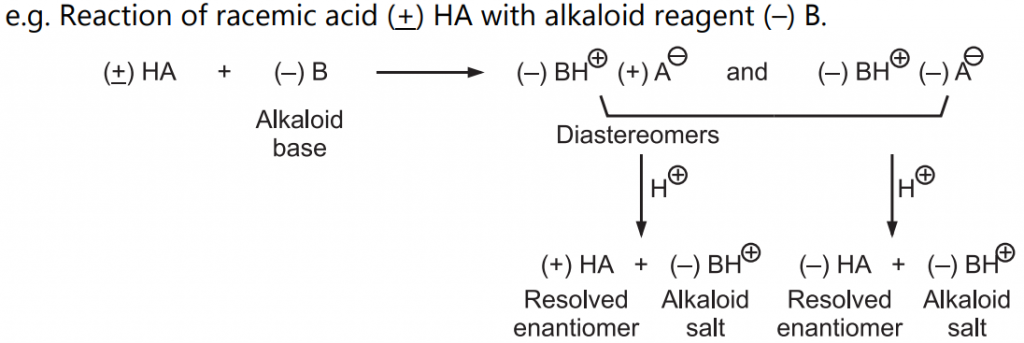
Formed diastereomers have different physical properties and can be easily separated by fractional distillation or crystallization. Further by the addition of mineral acid resolved enantiomers can be recovered from the solution.
Alkaloid bases commonly used are (–) brucine, (–) quinine, (–) strychnine, etc.
Similarly, racemic bases can be separated with acid reagents e.g. (–) malic acid. Compounds other than acids, bases can also be resolved. Alcohols are weakly ionized and are not appreciably acidic or basic so their resolution is facilitated by attaching them with an acidic handle which can be removed later.
4. Reactions where bonds with the chiral center are broken:
The stereochemistry of such reactions depends on the mechanism of the reaction. Hence, stereochemistry can be helpful to give evidence of a particular mechanism. e.g.
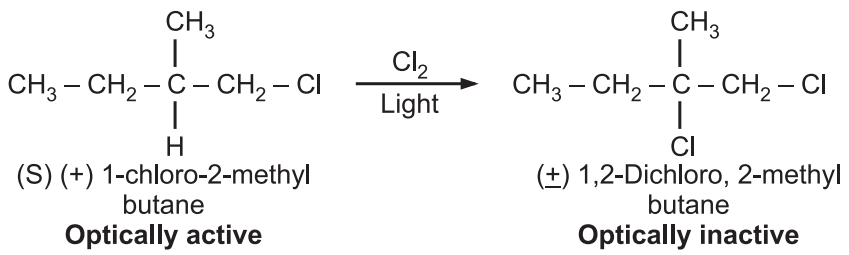
As the product is optically inactive and a racemic mixture, it implies second chlorine can be attached from either face of the intermediate, which can be a free alkyl radical with loss of chirality.
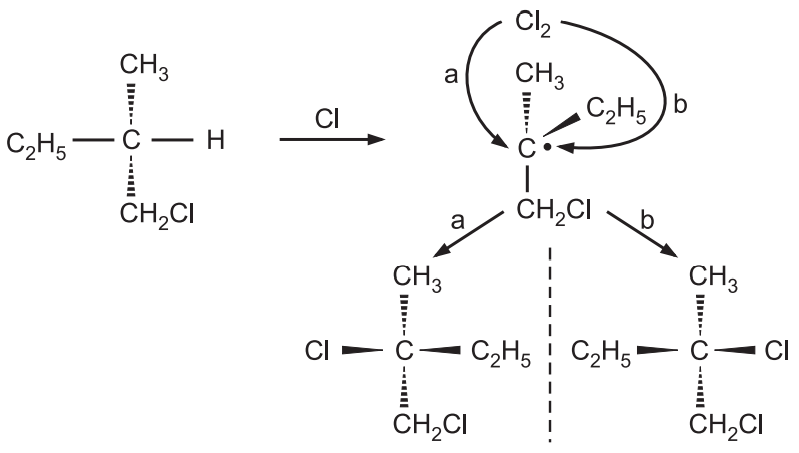
If there is a simultaneous attack of chlorine while the displacement of hydrogen, only the product from backside attack of chlorine would have been obtained instead of optically inactive product, so the mechanism involving free alkyl radicals is correct.
- A reaction is stereospecific when reactants exist as stereoisomers and each isomeric reactant gives a different stereoisomeric product.
- A reaction is stereoselective when reactant irrespective of any stereoisomerism produces predominantly or exclusively one stereoisomeric form of the product than other possible forms.
Make sure you also check our other amazing Article on : Elements of Symmetry