When drugs are in development, one property that is essential to their success is their solubility. Although water is widely used, most drugs being organic will not go into an aqueous solution easily. Strongly ionized substances are likely to be freely soluble in water over a wide pH range and cause no problem. Similarly, weakly acidic and weakly basic drugs should be sufficiently soluble at favorable pH. Sometimes soluble but the concentration of the solute is very close to its limit of solubility and gets precipitated on cooling or evaporation of the solvent. This section will briefly discuss ways to enhance the solubility of unionized drugs and weak electrolytes. There are several different ways to enhance solubility but the method of choice depends on the nature of the solute and the degree of solubilization needed.
Use of Cosolvents:
The cosolvent concept is used for increasing the solubility of electrolytes and non-electrolytes in water. This can be achieved by additional cosolvent that is miscible with water and in which the substance in question is soluble. The cosolvents work by modifying the affinity of solvent for solutes by a decrease in interfacial tension between solute and solvent or by changing the dielectric constant. The expected dielectric constant values for the solvent and cosolvent blend should be in the range of 25 to 80. Choice of such solvents for pharmaceutical use is limited due to toxicity and irritancy characteristics. Ethanol (for paracetamol), isopropyl alcohol (betamethasone valerate), glycerin, and propylene glycol (for co-triazole) are some of the examples of cosolvents used for the solubilization of drugs mentioned in brackets. Other examples of cosolvents are glycerin, polyethylene glycol, sorbitol, mannitol, etc., and are used for increasing solubilities of electrolytes and non-electrolytes.
pH Control:
The majority of the drugs are either weak acids or bases and therefore their solubilities in water can be influenced by the pH of the system. There is little or no effect of pH on the solubility of non-ionizable substances with few exceptions. For ionizable solutes such as carboxylic acid (HA) solubility is a function of pH, Fig.1. The solubility of a weak acid is increased by an increasing pH whereas solubility of a weak base is increased by decreasing pH.
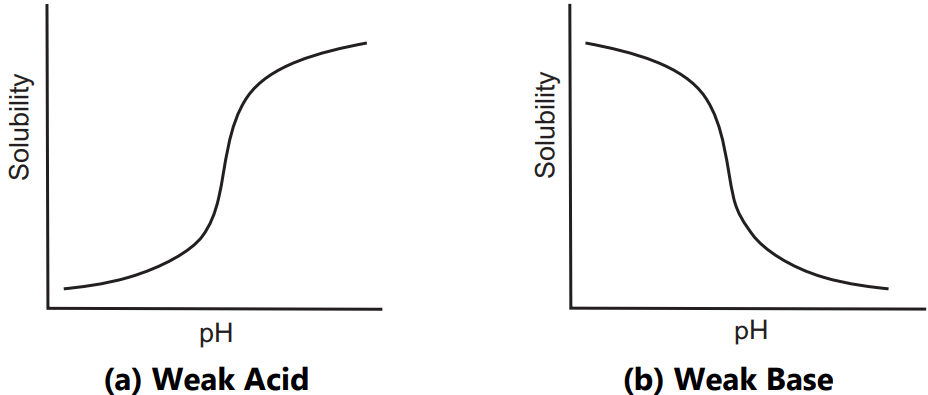
The pH of solute is related to its pKa and concentration of the ionized and unionized form of the solute by equation
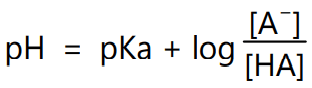
If the solute is brought outside its pKa by changing the pH value where the half portion is ionized and the half portion remains unionized, then the solubility will be changed. This is due to the introduction of intermolecular forces, mainly ionic forces of attraction. For example, carboxylic acid groups (−COOH) have pKa around pH 4 and if the pH is increased above 4 the −COOH is changed to −COO−. The negative charge introduced is free to have an introduction with a partial positive charge of the hydrogen of water. The effect of the pH on the solubility of weak electrolytes is described by the equation
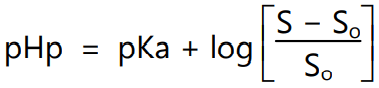
where pHp is the pH below which the drug precipitates from solution as the undissociated acid, S is the total solubility and So is the molar solubility of the undissociated acid. We often consider that ionize form is freely soluble but this is not always true. For example, carboxylic acids have pKa ~ 4. For the administration of methylprednisolone hemisuccinate (solubility <1 mg/ml) if a base such as sodium hydroxide is added the carboxylic acid becomes deprotonated and solubility increases to more than 200 mg/ml. The same can be observed for the base, therefore,
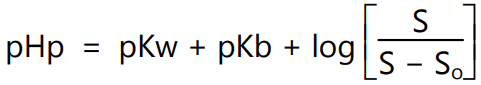
where pKw is the dissociation constant of water, pKb is the dissociation constant of base and pHp is the pH above which the free base precipitates out of solution.
The solubility of weak electrolytes in buffer solution can be changed by the addition of cosolvents. The undissociated species get dissolved by modifying the polarity of solvent to a more favorable value. In improving the solubility of drugs by pH control it must be ensured that the selected pH does not change the other requirement of the product such as chemical stability that may also depend on pH. Non-ionizable, hydrophobic solutes can have improved solubility by changing the dielectric constant of solvent by use of cosolvent. The maximum solubility must be best achieved by an appropriate balance between pH and the concentration of cosolvent. The solubilities of the non-electrolytes are not much affected by the pH changes therefore other methods can be tried for their solubility enhancement.
Surfactants in Solubilization:
Surfactants play a vital role in many processes of interest. One important property of surfactants is the formation of colloidal-sized clusters in solutions, known as micelles, which have significance in pharmacy because of their ability to increase the solubility of sparingly soluble substances in water. The solubility of drugs that are insoluble or poorly soluble in water can be improved by the incorporation of surfactants above their critical micelle concentration (CMC). This phenomenon is widely used for the solubilization of poorly soluble drugs. Micelles are known to have an anisotropic water distribution within their structure. In other words, the water concentration decreases from the surface towards the core of the micelle, with a completely hydrophobic (water-excluded) core. Consequently, the spatial entrapment of a solubilized drug in a micelle depends on its polarity. The non-polar molecules will be solubilized in the micellar core, and substances with intermediate polarity will be distributed along with the surfactant molecules in certain intermediate positions. Numerous drug delivery and drug targeting systems have been studied to minimize drug degradation and loss, prevent harmful side effects, and increase drug bioavailability. Within this context, the utilization of micelles as drug carriers presents some advantages when compared to other alternatives such as soluble polymers and liposomes. Micellar systems can solubilize poorly soluble drugs and thus increase their bioavailability, they can stay in the body (blood) long enough to provide gradual accumulation in the required area, and their sizes permit them to accumulate in areas with leaky vasculature.
In general, surfactants play an important role in contemporary pharmaceuticals, since they are largely utilized in various drug dosage forms to control wetting, stability, bioavailability, among other properties. It is important to notice that lyophobic colloids, such as polymers, require certain energy to be applied for their formation, are quite unstable from the thermodynamic point of view, and frequently form large aggregates. Association colloids such as micelles, on the other hand, can form spontaneously under certain conditions (self-assembling systems) and are thermodynamically more stable towards both dissociation and aggregation. Surfactants and their role in pharmacy are of paramount importance, especially for their ability of solubilizing hydrophobic drugs.
The hydrophilic surfactants having an HLB value above 15 such as sodium lauryl sulfate, polysorbates, polyoxyl stearate, polyethylene glycol, and castor oils are used for micellar solubilization. The fat-soluble vitamin phytonadione is solubilized by the use of polysorbates. The solubility of amiodarone hydrochloride can also be enhanced similarly. Macrogol ethers have been found to improve the solubility of iodine by producing ionophores. polyoxyethylene castor oil is used to increase the solubility of an immune expressing drug cyclosporine and anticancer drug paclitaxel. Cetomacrogol has been found to show improved solubility of chloramphenicol. The solubility of volatile and essential oils can be improved by the use of lanolin derivatives. Chloroxylenol which normally has a solubility of 0.03% in water can be improved by the use of soaps. Vitamin A, D, E, and K, griseofulvin, aspirin, and phenacetin, etc. are poorly soluble drugs that are solubilized by micellar solubilization.
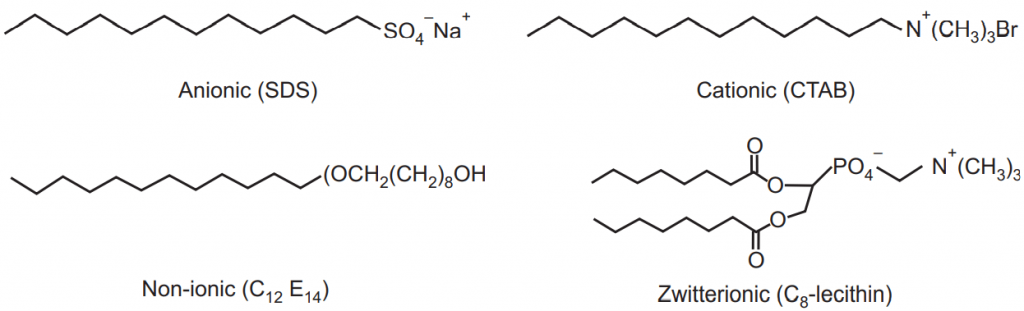
Surfactants are amphiphilic molecules composed of a hydrophilic or polar moiety known as the head and a hydrophobic or non-polar moiety known as the tail. The surfactant head can be charged (anionic or cationic), dipolar (zwitterionic), or non-charged (non-ionic). Sodium dodecyl sulfate (SDS), dodecyl tri-methyl ammonium bromide (DTAB), n-dodecyl tetra (ethylene oxide) (C12E4), and octanoyl phosphatidylcholine (C8-lecithin) are typical examples of anionic, cationic, non-ionic, and zwitterionic surfactants, respectively. The surfactant tail is usually a long chain hydrocarbon residue and less often a halogenated or oxygenated hydrocarbon or siloxane chain, Fig.2.
A surfactant, when present at low concentrations in a system, adsorbs onto surfaces or interfaces significantly changing the surface or interfacial free energy. Surfactants usually act to reduce the interfacial free energy, although there are occasions when they are used to increase it. When surfactant molecules are dissolved in water at concentrations above the CMC, they form aggregates known as micelles. In a micelle, the hydrophobic tails flock to the interior to minimize their contact with water, and the hydrophilic heads remain on the outer surface to maximize their contact with water, Fig.3. The micellization process in water results from a delicate balance of intermolecular forces such as hydrophobic, steric, electrostatic, hydrogen bonding, and van der Waals interactions.
The main attractive force results from the hydrophobic effect associated with the nonpolar surfactant tails, and the main opposing repulsive force results from steric interactions and electrostatic interactions between the surfactant polar heads. Whether micellization occurs and, if so, at what concentration of monomeric surfactant, depends on the balance of the forces promoting micellization and those opposing it.
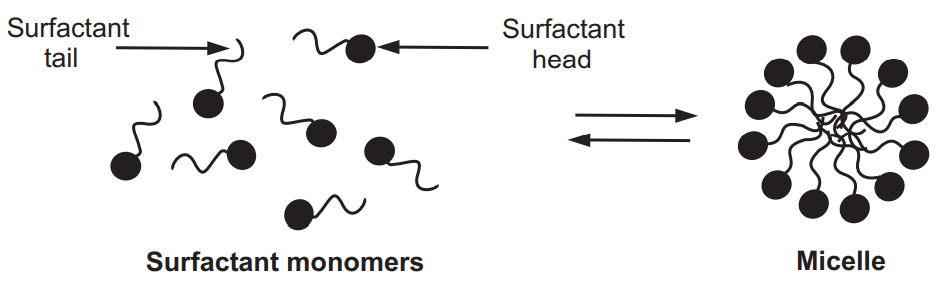
The dark circles represent the surfactant heads (hydrophilic part) and the black curved lines represent the surfactant tails (hydrophobic part).
Micelles are labile entities formed by the non-covalent aggregation of individual surfactant monomers. Therefore, they can be spherical, cylindrical, or planar (discs or bilayers). Micelle shape and size can be controlled by changing the surfactant chemical structure as well as by varying solution conditions such as temperature, overall surfactant concentration, surfactant composition (in the case of mixed surfactant systems), ionic strength, and pH. Depending on the surfactant type and on the solution conditions, spherical micelles can grow one-dimensionally into cylindrical micelles or two-dimensionally into bilayers or discoidal micelles. Micelle growth is controlled primarily by the surfactant heads, since both one-dimensional and two-dimensional growth requires bringing the surfactant heads closer to each other to reduce the available area per surfactant molecule at the micelle surface, and hence the curvature of the micelle surface.
A combination of solubilization and cosolvent can be used to increase the solubility of chloroxylenol. An alternative to the use of surfactants as solubilizing agents polymers such as cyclodextrins has been found to show improvement in the solubility of poorly soluble drugs such as itraconazole and corticosteroids.
Complex Formation:
The apparent solubility of some substance in the given solvent may be increased or decreased by the incorporation of complex-forming substances. The degree of complex formation decides the apparent change in solubility of the original solute. For example, complex formation between iodine and povidone increases the solubility of iodine. Similarly, the complex between iodine and potassium iodide to form polyiodides increases the solubility of iodine. The interaction of salicylates and benzoates with theophylline or caffeine also increases the solubility of these drugs. Other examples of complex-forming substances that increase the solubility of drugs are nicotinamide and β-cyclodextrins.
Drug Derivatization:
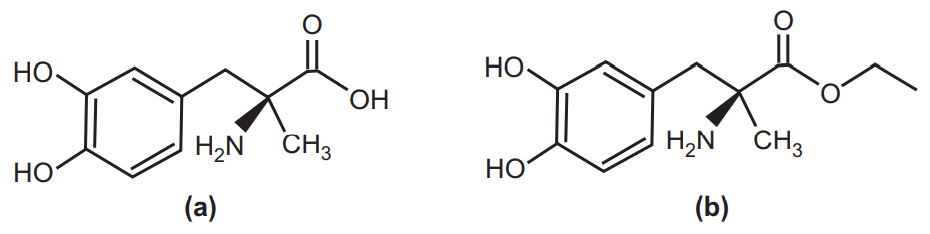
One method to increase the solubility of a drug is to alter the chemical structure of the molecule. The addition of polar groups like carboxylic acids, ketones, and amines can increase solubility by increasing hydrogen bonding and the interaction with water. Another structure modification can be to reduce intramolecular forces. An example of structure modification to enhance solubility by the latter method is methyldopa, solubility ~10 mg/ml, and methyl dopate (a prodrug of methyldopa), solubility 10-300 mg/ml depending on pH, Fig.4. The addition of the ethyl ester to methyldopa reduces the intramolecular hydrogen bond between the carboxylic acid and primary amine. Therefore, this addition reduces the melting point and increases solubility. Other examples of chemical modifications for the solubility enhancement include; sodium phosphate salt of hydrocortisone, prednisolone, and betamethasone.
Solid State Manipulation:
The size and shape of particles have a significant effect on solubilities. An increase in surface area by a decrease in particle size provides more area for interaction between solvent and solute causing higher solubilities.
Make sure you also check our other amazing Article on : HLB Scale