Fungus is a parasites. The human-fungi parasitic relationship results in mycotic illnesses, the majority of which involves superficial invasion of the skin or the mucous membranes of body orifices. Fungi have different shapes and sizes. Some are large while others are minute parasitic and saprophytic cells. They differ from
- algae in the lack of photosynthetic ability.
- protozoa in the lack of motility, possession of chitinous cell wall, and ease of culture on simple media.
- bacteria in greater size and in the presence of certain intracellular structures like mitochondria and nuclear membranes.
Depending upon some basic differences, fungi may be classified as:
(a) Phycomycetes (algae-like)
(b) Ascomycetes (sac-like)
(c) Basidiomycetes (mushrooms), and
(d) Dueteromycetes.
In addition, the ‘higher bacteria’ like Actinomyces and Nocardia are sometimes grouped with the fungi.
Fungal diseases are generally referred to as ‘mycoses’. Fungal infections (or mycoses) fall into two well-defined groups: superficial and deep-seated mycoses. These mycotic infections may be categorized broadly as:
(i) Dermatophytoses (skin infections) are contagious in nature and caused by various Trichophyton, Microsporum and Epidermophyton species. These include superficial infections of keratinized tissues like stratum corneum, hair, nails, etc. Tinea capitis, Tinea corporis, Tinea cruris, Tinea unguium, Tinea versicolor, Tinea nigra and candidiasis are all grouped under superficial fungal infections. As a rule, these lesions are mild, superficial, and restricted. The causative microbes are specialized saprophytes with the unusual ability to digest keratin. They have an ultimate reservoir in the soil. Topical antifungal agents are effective here. However, to treat deeper infections systemic antifungal (e.g. griseofulvin) may also be given along with topical antifungal agents. Because of the keratolytic action of salicylate, it may often be used along with the topical antifungal agent to improve drug penetration. Salicylic acid helps the drug reach the site deep within the hyperkeratotic epidermis.
(ii) Candidiasis affects mainly the skin and mucous membranes. It is caused by Candida albicans. These infections mainly develop in the mouth, bowel, or vagina and are called as local infections. They may sometimes become systemic and contagious.
Table.1: Drugs of choice in the treatment of systemic fungal infections
| Sr.No. | Disease | Fungus | Effective antifungal agents |
| 1 | Actinomycosis | Actinomyces israelii | Penicillin G, cephalosporin, tetracycline |
| 2 | Aspergillosis | Aspergillus fumigatus Aspergillus niger | Amphotericin B, rifampin |
| 3 | Blastomycosis (North American type) | Blastomyces dermatitidis | Amphotericin B, rifampin and hydroxystibamidine, itraconazole |
| 4 | Blastomycosis (South American type) | Blastomyces brasiliensis | Amphotericin B, miconazole |
| 5 | Candidiasis | Candida albicans | Amphotericin B, nystatin, flueonazole |
| 6 | Chromoblastomycosis | Cladosporium | Flucytosine, amphotericin B, potassium iodide |
| 7 | Coccidioidomycosis | Coccidioides immitis | Amphotericin B, fluconazole miconazole, ketoconazole |
| 8 | Cryptococcosis | Cryptococcus neoformans | Amphotericin B, flucytosine, fluconazole |
| 9 | Histoplasmosis | Histoplasma capsulatum | Amphotericin B, hydroxystilbamidine, rifampin |
| 10 | Phycomycosis (Mucormycosis) | Mucor species | Amphotericin B, hydroxystilbamidine |
| 11 | Nocardiosis | Nocardia asteroides | Amoxicillin, cotrimoxazole, minocycline |
| 12 | Paracoccidioidomycosis | – | Ketoconazole, amphotericin B |
| 13 | Sporotrichosis | Sporothrix schenckii | Potassium iodide, amphotericin B, itraconazole |
| 14 | Fusariosis | – | Amphotericin B |
| 15 | Pseudoallescheriosis | – | Ketaconazole, itraconazole |
| 16 | Zygomycosis | – | Amphotericin B |
Itraconazole is a 1: 1: 1: 1 racemic mixture of 04 diastereomers, each possessing three chiral centers.
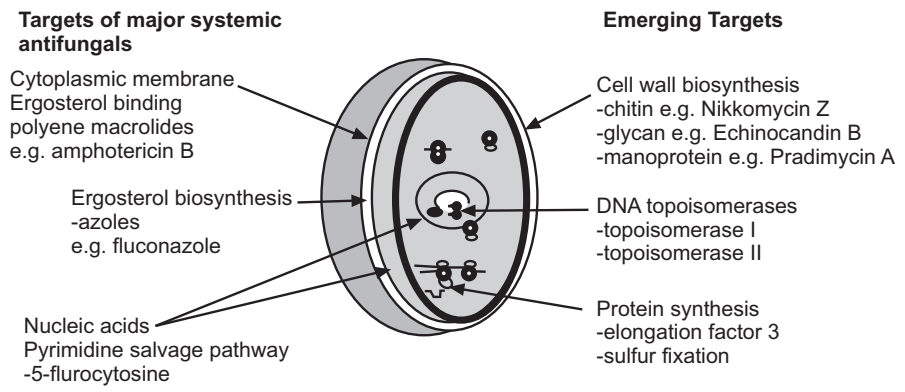
(iii) Systemic fungal infection (see Table 1), is the third major category that involves fungal infections of bones, viscera, lungs, and meninges. Many fungal infections occur either on the skin (avascular region) or in poorly vascularized areas (e.g. nails and hair). At such places, the drug cannot build up its therapeutic concentration necessary to exhibit antifungal activity. Besides this, the clinical utility of many drugs is hampered mainly because of poor solubility and poor penetration ability. Currently, there exist neither clinically available vaccines nor effective antisera for mycotic diseases. Due to various reasons (e.g. differences in solubility, diffusibility, or inactivation by serum components), the agents showing excellent antifungal activity in-vitro studies, disappointed us when tested in-vivo. For example, miconazole and clotrimazole are inactivated by phospholipids and triglycerides. To cover such a broad range of systemic fungal infections, very few antifungal agents are available. These include polyene antibiotics (e.g. amphotericin B, nystatin), antimetabolites (e.g. flucytosine), griseofulvin, and imidazoles (e.g. ketoconazole, miconazole, and clotrimazole).
Regardless of the type of fungus that is causing an infection, treatment is extremely difficult because fungi, like mammalians, are eukaryotes. Many biochemical structures, especially cell membranes, are nearly identical, as are many biochemical reactions.
In human cells, the sterol in the membrane is cholesterol. In fungi, the sterol is ergosterol. This difference is the only source of selectivity that we have in treating fungal infections.
Classification:
On a chemical basis, currently used antifungal agents can be categorized as:
- Fatty acids
- Pyrimidine derivatives
- Imidazoles
- Allylamines
- Amidines
- Antifungal antibiotics
- Miscellaneous agents.
Due to the diversified structures of various antifungal agents, attempts to define SAR also failed. In such cases, interpretation of the activity in terms of ‘drug physicochemical parameters’ projects a better understanding of SAR studies.
(a) Fatty Acids: In 1939, Peck reported that sweat has antifungal properties. The antifungal activity of ingredients of perspiration has nothing to do with the pH of the perspiration and the activity is attributed to the presence of fatty acids and their salts. Propionic acid, undecylenic acid, and sodium caprylate are effective against infections due to Trichophytons, Microsporons, and Candida albicans. The recognition of their antifungal activity led to their clinical use mainly in the form of sodium and zinc salts. For example, the mixture of sodium propionate and propionic acid may be used in the treatment of ringworm while undecylenic acid and zinc undecylenate, in the form of a mixture, are effective against ringworm and monilial vaginitis. Effectiveness increases when zinc salts of these acids are used. They are applied in the form of an ointment, lotion, or dusting powder. However, their application to eyes, ears, nose or other areas of the mucous membrane should be avoided. Their antifungal activity is related to their ability to precipitate fungal proteins. Heavy metal ions like Ag+, Hg++, Cu++, and Zn++ combine with the functional groups present on the surface of enzymes. Hence, when combined with an organic molecule, they potentiate the antifungal activity of the latter.
(b) Pyrimidine Derivatives: 5-Fluorocytosine is a fluorinated pyrimidine and is related in structure to fluorouracil and flouridine. First introduced in 1957, it failed to build up its career as an effective antineoplastic drug. Ten years thereafter when tested for antifungal activity, it proved its potential against Candida species and Cryptococcus neoformans infections.
The antifungal activity of 5-fluorocytosine is attributed to the formation of 5-fluorouracil (an active metabolite) from the drug by fungal cytosine deaminases.
The active metabolite is further converted to 5-fluro-2′-deoxyuridylic acid which interrupts the fungal DNA synthesis by inhibiting thymidylate synthetase enzymes. Since mammalian cells do not contain cytosine deaminase, their function is not affected by flucytosine. Besides this, it is also suspected to interfere with protein synthesis.
It is not used topically. When used orally, about 4% of an administered dose is bound to the plasma proteins. It has a plasma half-life of 4.2 – 4.5 hours. It rapidly deaminates in fungal cells to the antimetabolite, 5-fluorouracil. About 80% of the dose appears in the urine in an unchanged form.
Adverse effects include nausea, vomiting, diarrhea, enterocolitis, headache, skin rashes, vertigo, anemia, sedation, hepatomegaly, hepatic necrosis, leukopenia, agranulocytosis, and thrombocytopenia.
Flucytosine is effective against infections caused by C. neoformans, C. albicans, T. glabrata and S. schenckii. It is also effective against chromomycosis caused by Cladosporium species and Phialophora species. It is used in combination with amphotericin B for the treatment of infections due to yeasts and yeast-like fungi. Amphotericin renders the yeast cell membrane more permeable to flucytosine. Hence, both drugs are used in the treatment of cryptococcal meningitis, Candida endocarditis, and pulmonary and urinary tract infections. It may also be used in the treatment of chromoblastomycosis.
(c) Imidazoles: Imidazole derivatives are associated with many therapeutic fields. Some have been employed as anthelmintics. Antibacterial and antiprotozoal activities also have been observed in some analogs.
The first azole to become available for clinical use (as a topical agent) was chlormidazole, introduced by Chemie Gruenenthal in 1958. It was followed by the introduction of miconazole by Janssen in 1969, clotrimazole by Bayer’s. Later on, a new imidazole, econazole was launched by Janssen in 1974. Even today, the latter three agents remain the mainstay of topical therapy for many dermatophytoses.
Miconazole, clotrimazole, ketoconazole, econazole, itraconazole, fluconazole, tioconazole, bifonazole and terconazole are some currently used antifungal imidazole derivatives. They all have activity against a broad range of microorganisms including both fungi and bacteria. Clotrimazole, econazole and tioconazole are effective against superficial fungal infections while bifonazole and terconazole are effective in vulvovaginal candidiasis. Other imidazoles like, ketoconazole and miconazole are effective against both, superficial and systemic infections.
Imidazole derivatives act by damaging the fungal cell membrane. They enhance the membrane permeability by inhibiting the synthesis of ergosterol which is the primary cellular sterol of fungi.
Clotrimazole shows poor oral absorption. Whatever amount is absorbed, gets rapidly inactivated by cytochrome P-450 enzymes in the liver. It is a broad-spectrum antifungal agent having fungistatic activity against dermatophytes, C. albicans, C. neoformans, S. schenckii and B. dermatitidis. It is used topically in 1-2% concentration as cream, lotion and vaginal preparation to treat cutaneous candidiasis, vulvovaginal candidiasis, and dermatophyte infections. It may sometimes be combined with an antibacterial agent in some treatment regimen. Miconazole is a potent antifungal imidazole derivative that may be used topically, intravenously, or intrathecally. About 90% of the administered dose is bound to the plasma proteins. It has a plasma half-life of 24 hours and is extensively metabolized in the liver.
The azole class of antifungals acts by damaging the fungal cell membrane. These drugs selectively inhibit the biosynthesis of ergosterol by inhibiting fungal cytochrome P-450 sterol 14α-demethylase. Azoles thus inhibit demethylation at C14 of lanosterol thereby causing accumulation of 14α-methylated sterols that disrupt the various sterol functions in the cell. This mode of action results in the inhibition of fungal cell growth but does not bring about the death of the fungal cell. Hence, the azoles are fungistatic.
Adverse effects include nausea, vomiting, headache, blurred vision, skin rash, burning, itching, irritation, weakness, arthralgia, seizures, confusion, anemia, and thrombocytopenia.
It is effectively used topically in the treatment of tinea pedis, tinea cruris, tinea versicolor, onychomycosis, cutaneous candidiasis, pruritus, and other superficial dermatomycoses (2% cream, spray, powder or lotion may be applied topically twice a day for 1-2 weeks). To treat vulvovaginal candidiasis and vaginal infections caused by T. glabratus, it may be used in the form of a 2% vaginal cream or 100 mg suppositories. The latter may be applied deep in the vagina at bed time for 7 days and in the case of 200 mg vaginal suppositories, a three-day treatment is usually advised.
Parenterally it may be used to control systemic fungal infections like coccidioidomycosis, paracoccidioidomycosis, cryptococcosis, systemic candidiasis, and mucocutaneous candidiasis. In patients with coccidioidal meningitis and urinary bladder infections, the I.V. route must be supplemented by intrathecal and intrabladder irrigation routes respectively. The free base may also be used topically to treat ophthalmic mycoses.
Ketoconazole is an orally active, broad-spectrum antifungal agent that is chemically related to miconazole. About 99% of the administered dose is bound to the plasma proteins. It has a plasma half-life of 2 – 4 hours. It is extensively metabolized in the liver primarily by oxidative o-dealkylation and aromatic hydroxylation to various inactive metabolites that are excreted (85 – 90%) in the bile. It serves as an effective antifungal agent in the treatment of mucocutaneous candidiasis, vaginitis, oral thrush, blastomycosis, coccidioidomycosis, nonmeningeal cryptococcal disease, histoplasmosis, and some dermatomycoses. It may also be concomitantly administered with flucytosine in the treatment of cryptococcal meningitis.
Econazole nitrate is used topically for the treatment of superficial fungal infections of the skin. While tioconazole is used in the treatment of dermatophyte infections and candidiasis.
From the many series of azoles that have been reported by diverse groups, several common structural features emerge: an imidazole or triazole heme-coordinating group, a halo substituted aromatic ring separated from the azole moiety by two atoms, and a side chain. The latter represents the feature of the greatest diversity across the family.
The variable length of the side chain explored by various groups suggests that this part of the pharmacophore may extend beyond the substrate binding site, perhaps into the substrate access channel.
(d) Allylamines: The allylamines are the most prominent of a number of antifungal classes that exert their activity by inhibition of squalene epoxidase; the intracellular accumulation of squalene that results is thought to be the primary cause of the fungicidal consequences of exposure to the drug. The predominant example of this class of antifungal agents is terbinafine, which is one of the main drugs for the treatment of dermatophytosis.
The inhibition of squalene epoxidase by the allylamines is reversible and noncompetitive with respect to squalene, NADPH, and FAD and the agents have no effects on other enzymes in the ergosterol biosynthetic pathway.
The structural requirements for potent activity are represented in the broadest sense by two lipophilic domains linked to a central polar moiety by a spacer of appropriate length; for good activity, the polar moiety is a tertiary amine, and one of the lipophilic domains consists of a bicyclic aromatic ring system such as naphthalene or benzo [b] thiophene.
(e) Amidines: In this category, hydroxystilbamidine and stilbamidine represent examples of effective antifungal agents. These agents are active against fungi and protozoa. Generally, they are used in the treatment of cutaneous blastomycosis, actinomycosis, and cryptococcosis.
Hydroxystilbamidine disethionate is administered only by the I.V. route. Nothing is known about its bio-transformation and excretion. Adverse effects include nausea, vomiting, diarrhea, anorexia, rash, fever, chills, anaphylaxis, headache, malaise, fainting, hypotension, dizziness, pancreatitis, hypoglycemia, and hepatotoxicity. It is used in the treatment of cutaneous and pulmonary blastomycosis and visceral leishmaniasis.
A solution of 225 mg in 200 ml of 5% dextrose water is freshly prepared and is given by I.V. infusion over a period of 2-3 hours every 24 hours.
(f) Antifungal Antibiotics:
(i) Griseofulvin: It is isolated in 1939 by Oxford, Raistrick, and Simonart. Since it was ineffective against bacteria, its appearance on the clinical screen was delayed by almost about 20 years merely due to ignorance about its antifungal activity. It is obtained from the yeast, Penicillium griseofulvum.
Gentles in 1958, first reported its antifungal activity. Griseofulvin affects only fungi with chitinous cell-wall. The drug is fungistatic rather than fungicidal. It is ineffective in the treatment of systemic mycoses. It may be used orally or topically in the treatment of superficial mycoses of skin, hair, and nails caused by most strains of Microsporum, Trichophyton, and Epidermophyton. It does not have an effect on bacteria, yeasts, or other fungi. Its antifungal activity is mainly due to its interaction with the polymerized microtubules. Microtubules are the protein structures found in eukaryotic cells that are responsible for the formation of mitotic spindles. The drug-induced disruption of mitotic spindles slows down oxidative phosphorylation and nucleic acid synthesis in the fungal cells.
Griseofulvin is nil bound to the plasma proteins. However, it specifically binds to keratin and high concentrations are found in the stratum corneum. A significant concentration of a drug is also retained by the nails. It has a plasma half-life of about 24 hours. The major metabolite is 6-methyl-griseofulvin which is excreted in the urine. Some fraction also appears unchanged in the feces.
Adverse effects include nausea, vomiting, diarrhea, headache, blurred vision, dry mouth, fatigue, lethargy, peripheral neuritis, heartburn, erythema, albuminuria, leucopenia, and hepatotoxicity. It is contraindicated during pregnancy and in patients with porphyria and liver failure.
Besides its effectiveness in mycotic infections, it also showed promising results in lichen planus, anginal attacks, and Raynaud’s syndrome.
(ii) Polyene antibiotics: In the early 1950s, polyene antibiotics were first identified. As the name implies, these compounds contain unsaturated carbon rings or chains. About 60 polyene antibiotics (all produced by actinomycetes) have been reported in the literature.
All polyenes are characterized by the presence of a large ring containing a lactone group (i.e. macrocyclic lactone) and a hydrophobic region coupled with a conjugated polyene system of four to seven double bonds. Many of them contain glycosidically linked amino sugars. For example, an aminodesoxy hexose (i.e. mycosamine) is present in amphotericin B and nystatin. Polyene antibiotics are poorly soluble in water. The number of double bonds present in the skeleton serves as a basis for the classification of polyenes. For example, they may be categorised as tetraenes (nystatin), pentaenes (filipin), hexaenes (endomycin), and heptaenes (amphotericin B). The most important polyenes are amphotericin B and nystatin. The former is an important therapeutic agent against most systemic antifungal diseases. Depending upon the concentration employed, polyene antibiotics exert either fungistatic or fungicidal effects.
These polyene macrolides preferentially bind ergosterol, the predominant fungal sterol, resulting in a permeable plasma membrane and rapid cell death. However, amphotericin also binds to the cholesterol of the mammalian cytoplasmic membrane. This results in the alteration of the mammalian cytoplasmic membrane and may explain the severe nephrotoxicity accompanying the use of amphotericin B.
(iii) Amphotericin B: Amphotericin is a mixture of two compounds A and B, obtained from Streptomyces nodosus, a soil actinomycete reported in 1956. As the name suggests, amphotericins are amphoteric in nature. Amphotericin B is more potent and it possesses a broad spectrum of activity. It is effective against Aspergillus fumigatus; B. dermatitidis, Candida species, C. neoformans, H. capsulatum, Coccidioides immitis, M. audouinii, Paracoccidiodes brasiliensis, Rhizopus species, Rhodotorula species, Sporothrix schenckii, Torulopsis glabrata, Trichophyton species, and Mycobacterium leprae. It exerts maximum antifungal effect between the pH range of 6.0 – 7.5.
It is available in the form of a mixture, lozenges, and ointment. It has a poor oral absorption pattern. It is effective against a number of fungal infections including aspergillosis, blastomycosis, candida infections, leishmaniasis, histoplasmosis, sporotrichosis, and coccidioidomycosis. It is used topically to treat external ocular infections (i.e. mycotic conjunctivities). For topical use, amphotericin B is available in a 3% concentration as a cream, lotion, and ointment. It may be used intravenously or subconjunctivally in the treatment of fungal corneal ulcers or endophthalmitis. In cryptococcal meningitis, it is usually combined with 5-fluorocytosine to get a synergistic action. It may also be given intra-articularly, especially in sporotrichosis and coccidioidomycosis.
In many occasions, flucytosine, minocycline, or rifampin, in fact, may be added to the regimen in order to reduce the minimum antifungal concentration of amphotericin B. Since the latter is one of the most toxic antimicrobial agents which is in use today, the reduction in the dose of amphotericin B, helps in improving the patient’s comfort. Amphotericin B methyl ester has equipotent antifungal activity but has better pharmacokinetic features. However, it could not reach the market because of its ability to cause leukoencephalopathy.
(iv) Nystatin: Nystatin was first isolated from Streptomyces noursei in 1951 by Hazen and Brown. The name of this antibiotic was derived from New York state where it was discovered. It is only slightly soluble in water and is unstable to moisture, heat, light, and air. It exerts no effect on bacteria, protozoa, or viruses. When used topically, nystatin may sometimes be combined with iodochlorhydroxyquin.
It is effective specifically against Candida, Microsporum, Trichophyton, Leishmania, B. dermatitidis, C. neoformans, H. capsulatum, T. vaginalis and dermatophytes. It is often combined with gention violet, procaine hydrochloride, antibiotics or hydrocortisone and is used topically in the treatment of candidiasis of the skin, mouth, intestine, conjunctival sac, nails, and vagina. It may be given as aerosol or instilled into the conjunctival sac. However, it should not be used parenterally due to severe systemic toxicities.
Natamycin is yet another tetraene antifungal antibiotic obtained from Streptomyces natalensis in 1958. It is a broad spectrum antifungal agent specifically effective against occular infections caused by Fusarium solani and Myceliating fungi. It is used to treat fungal keratitis, fungal blepharitis, and fungal conjunctivitis. It may also be inhaled into the respiratory tract to cure broncho-pulmonary aspergillosis and candidiasis.
Hamycin and candicidin are examples of other antifungal polyene antibiotics. Candicidin was isolated in 1953 from Streptomyces griseus and is used topically in the treatment of vaginal candidiasis. Hamycin is isolated from Streptomyces pimprima and is effective against Blastomyces dermatitidis, Histoplasma capsulatum, Aspergillus fumigatus, Cryptococcus neoformans, and Candida albicans. It may be used topically to control vaginal candidiasis.
(g) Miscellaneous Agents: (i) Tolnaftate: Tolnaftate is a topical antifungal agent available as a cream, solution, powder, and aerosol in 1% concentration. It is a thiocarbamate derivative and is used in the treatment of cutaneous mycoses or ringworm infections. When used against other fungal or bacterial infections, it remains ineffective.
Like the allylamines, it is a non-competitive inhibitor of squalene epoxidase, with inherent selectivity for the fungal enzymes over mammalian. It is topically applied to the affected area twice a day. However, relapses may occur after cessation of therapy.
(ii) Cyclopirox olamine: It is a topically used broad-spectrum fungicidal agent effective against dermatophytes. Its antifungal activity may be related to its ability to interfere with the uptake of precursors needed for the synthesis of proteins and nucleic acid core. Adverse effects are few and include pruritis and burning at the site of application.
(iii) Potassium iodide: In the form of a saturated solution of 1 g/ml, it may be used orally as an effective antifungal agent in the treatment of cutaneous and lymphatic forms of sporotrichosis, caused by S. schenckii. It is excreted in the urine. It probably acts by the iodination of proteins in the fungus cell membrane. Adverse effects include nausea, vomiting, diarrhea, heart-burn, sneezing, shedding tears, metallic taste, acneiform skin lesions, and swelling of the parotid gland. Iodism is frequently encountered during therapy.
Whitfield’s Ointment: It contains a mixture of benzoic acid (fungistatic agent) and salicylic acid (keratolytic agent). The keratolytic action of salicylic acid helps for desquamation of stratum corneum. This promotes the removal of offending fungus resulting in better penetration of antifungal agents.
Fungal Cell Wall: The cell wall is one of the most attractive targets in the fungal cell. It morphologically defines and protects the cell from lysis and its continued biosynthesis is essential for growth and survival. It is composed primarily of three polysaccharides; β-1, 3-glucan, chitin (β-1, 4-N-acetylglucosamine) and mannoprotein (largely α-1, 6-mannose). None of the major components of the cell wall or enzymes involved in their biosynthesis occurs in mammalian cells.
Table.2: Clinically used antifungal agents
| SL.NO | Drug | Uses | Possible mechanism of action |
| 1 | Polyene antibiotics | Candidiasis, Histoplasmosis, Blastomycosis, Sporotrichosis | Interact with fungal membrane sterols and change the selective permeability of the fungal membrane. |
| 2 | Griseofulvin | T. capitis, T. circinata, T. pedis, Onochomycosis | Interacts with fungal microtubules and prevents cell division. |
| 3 | Imidazole derivatives | Cryptococcosis, Histoplasmosis, Mucormycosis | Inhibits ergosterol synthesis in fungal cell membrane resulting in leakage of cell constituents. |
| 4 | Flucytosine | Candidiasis Aspergillosis | Inhibits the formation of fungal nucleic acids. |
| 5 | Tolnaftate | Cutaneous mycoses | Inhibits transport of precursors for proteins and nucleic acid in fungi. |
| 6 | Ciclopirox | Cutaneous mycoses | Inhibits the transport of precursors needed for the synthesis of proteins and nucleic acid in fungi. |
| 7 | Potassium iodide | Sporotrichosis | Iodination of proteins in the fungal cell membrane. |
Antibiotics: Many antibiotics like, minocycline, tetracyclines, penicillines, and rifampicin may be administered concomitantly with antifungal agents. Though they do not have antifungal potential, they can enhance the activity of antifungal agents (e.g. amphotericin B) when used in combination. Beside them, pyrrolnitrin, variation, and siccanin are other examples of antibiotics used in mycotic diseases.
Plant Products: Many workers have described plant products as effective antifungal agents in the treatment of skin disorders. However, the reports on the antifungal properties of essential oils are quite meager and fragmentary.
Other Organic Compounds: Salicylic acid, aminacrine, acrisorcin, halopragin, p-chloro-meta xylenol, salicylamide, salicylanilide, iodochlorhydroxyquin, m-cresyl acetate, diamethazole, chlordantoin, gention violet, pecilocin, di-iodohydroxy quinoline, iodine, phenyl mercuric nitrate, thymol and zinc pyrithione possess significant antifungal activity. Most of them may be used in the treatment of ringworm infections of scalp, feet, and groin. Haloprogin is a synthetic iodinated trichlorophenol available as a 1% cream. It is effective against various candidial and ringworm infections of the skin. Thymol in 1 – 2% concentration may be added to Whitfield’s ointment to enhance its antifungal potential. Zinc pyrithione may be used as a 1% solution to control infections due to the Pityrosporum ovale and Tinea versicolor. While salicylanilide may be used along with undecylenic acid in the form of 5% ointment to exhibit antifungal activity.
Natural Products: The various fungal organisms produce antibacterial substances along with such antifungal agents which do not exhibit significant antibacterial properties. Such types of anti-fungal antibiotics include pyrrolnitrin, variation, siccanin, and natamycin.
Make sure you also check our other amazing Article on : What Are The First-Line Agents For Tuberculosis?
