Molecular formula: C6H5COONa
Molecular Weight: 144.11
Material required: Sodium benzoate: 0.6 g, glacial acetic acid: 100 mL, 0.1N perchloric acid and crystal violet.
Procedure: Weigh accurately about 0.6 g of Sodium Benzoate, previously dried at 110°C and dissolved in 100 mL of glacial acetic acid, add 2 drops of crystal violet as an indicator, and titrate with 0.1N perchloric acid. Perform blank determination and make necessary corrections.
Reaction:
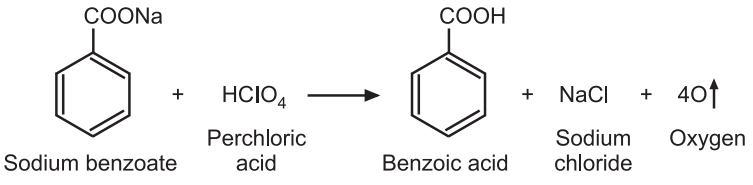
Factor Calculation:
1 Mole of HClO4 ≅ 1 Mole of sodium benzoate
1000 mL of 1M HClO4 ≅ 144.11 g of C6H5COONa
1 mL of 0.1N HClO4 ≅ 0.01441 g of C6H5COONa
Make sure you also check our other amazing Article on : Assay of Methyldopa