Preparation and Standardization of Sodium Hydroxide
Aim: To perform the standardization of sodium hydroxide.
Requirements:
- Apparatus: Beaker, Funnel, Pipette, Burette.
- Chemicals: Sodium hydroxide, Potassium hydrogen phthalate, Phenolphthalein solution.
Theory:
Titration is a process in which a standard solution is added in a controlled manner to the solution which is to be analyzed.
The acid-base titration is based on neutralization reaction and hydrogen ion indicators or pH indicators. This indicator shows colour change according to the concentration of hydrogen ions or pH.
In this titration generally, water-soluble indicators are available commercially and are a great convenience. The transition in colour is generally affected by temperature, the concentration of neutral salt in solution, the concentration of indicators.
Theory of Indicators: Acid-base indicator shows different colours in acidic solution and different in basic solution. Two theories are given below to explain the colour change. (a) Ostwald’s theory is based on ionisation (b) The Quinoid theory
(a) Ostwald’s Theory based on Ionisation:
According to this theory, the colour of the solution is due to the presence of coloured ions present in water. Indicators in the solution can either act as a weak acid or weak base that on dissociation have a different colour from the parent ion. e.g. Phenolphthalein.
Phenolphthalein is a weak acid whose undissociated molecule is colourless. On dissociation, it gives proton ion which is also colourless and rest molecule possesses deep pink colour.
HPh → H+ + Ph–
As the concentration of hydrogen ion increases, dissociation of HPh decreases up to nill. So the solution is colourless. In addition to alkali (NaOH), it gives OH– ion which combines with H+ to form an ionized water molecule. As water molecule is formed, equilibrium gets disturbed and phenolphthalein undergoes ionization. Phenolphthalein on ionization form sodium salt of phenolphthalein which shows colour (pink).
HPh → H+ + Ph−
NaOH → Na+ + OH−
Ph− + Na+ → NaPh (Pink)
H+ + OH− → H2O
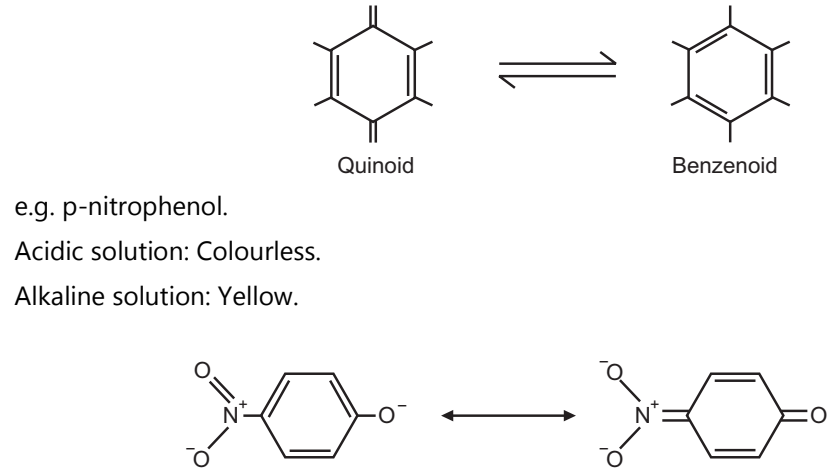
(b) Quinoid Theory:
According to this theory, the chromophore is responsible for imparting the colour change of the compound.
Chromophore means colour carrier. e.g. C=C, C=O, N=N.
Quinoid theory is based on intramolecular changes. It believes that indicator contains a mixture of two forms of indicator, one in acidic solution and the other in basic solution. These two forms have a different colours. At the endpoint, it transforms from one form to another and shows the colour.
Principle:
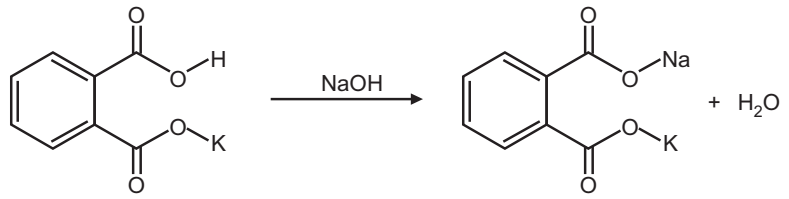
The reaction between an acid and a base produces salt and water, something that you may have heard before. In the reaction that you will be investigating, the acid is potassium hydrogen phthalate (KHP), and the base sodium hydroxide.
(NaOH) · NaOH(aq) + KHC8H4O4 (aq) = KNaC8H4O4(aq) + H2O(l)
As long as a reaction goes to completion, if one knows the following information:
- The balanced chemical equation for the reaction occurring.
- The exact quantity of one of the reagents (the KHP, a white solid, the mass of which you will weigh) then one can always determine the exact amount of any other substance involved in the reaction. This is the essence of the technique called quantitative chemical analysis.
In this experiment, we are going to determine the concentration of a sodium hydroxide solution by adding it to a known amount of KHP. The process used to carry out this addition is called titration. This is where one of the reagents (in our case NaOH) is added slowly from a burette to a known quantity of the other reagent (KHP). The point at which sufficient reactant has been added to just complete the reaction is called the equivalence point. This is what we would like to determine since at this point we have added a sufficient amount (moles) of NaOH to react with the entire amount (moles) of KHP. A method to determine this visually is to add a dye (referred to as an indicator) that changes colour at or extremely close to this point. The point at which the indicator changes colour is referred to as the endpoint. As far as we are concerned at this stage, the endpoint and the equivalence point are the same. The criteria used for choosing an indicator in a particular titration is, how close the indicator’s endpoint (where it changes colour) is to the equivalence point of the titration.
Procedure:
(A) Preparation of sodium hydroxide (1M): Dissolve 42 g of sodium hydroxide insufficient carbon dioxide-free water to produce 1000 ml.
(B) Standardisation: Weigh accurately about 5 g of potassium hydrogen phthalate previously powdered and dried at 120°C for 2 hours, and dissolved in 75 ml carbon dioxide-free water. Add 0.1 ml phenolphthalein solution and titrate with the sodium hydroxide solution until a permanent pink colour is produced. 1 ml of 1 M sodium hydroxide is equivalent to 0.2042 g of potassium hydrogen phthalate.
Observation Table of Preparation and Standardization of Sodium Hydroxide
| Sr. No. | Parameters | Reading |
| 1. | Burette solution | NaOH solution |
| 2. | Conical flask solution | Potassium hydrogen phthalate, water |
| 3. | Indicator | Phenolphthalein |
| 4. | Endpoint | Colourless to pink |
| Burette reading (BR) (ml) | Trial .1 | Trial .2 | Trial .3 | Average (BR) |
Reaction:
Calculations:
(A) Factor Calculation:
- Molecular weight of KHP = 20.42 g.
- From the reaction,
- 1 mol of NaOH is equivalent to 1 mol of KHP.
- Hence, 1000 ml of 1 M NaOH is equivalent to 20.42 g of KHP.
- So, 1 ml of 1 M NaOH is equivalent to 0.2042 g of KHP.
(B) Determination of Normality:
- 1 ml of 1 M NaOH is equivalent to 0.2042 g of KHP.
- So, (B.R) ml of A M NaOH is equivalent to 5 g of KHP.
- Hence, A = 1 × 5 /0.2042 × B.R
Result: The normality of given NaOH was found to be “A” N.
Make sure you also check our other amazing Article on : Limit Test for Iron
