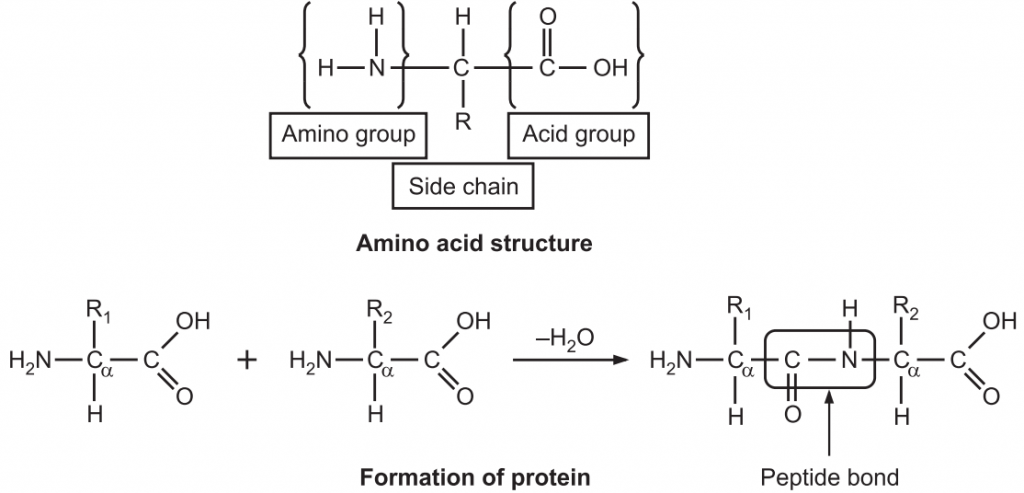
Protein was first described by the Dutch chemist Gerhardus Johannes Mulder and further named by the Swedish chemist Jons Jakob Berzelius in 1838. The term protein, derived from the Greek proteios, meaning first, is a class of organic compounds that are present in and vital to every living cell. Proteins are large biochemical compounds (carbon, hydrogen, oxygen, and nitrogen) consisting of one or more polypeptides (amino acid residue) typically folded into a globular or fibrous form in a biologically functional way. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of adjacent amino acid residues i.e. an amine group (NH2), a carboxylic acid group (R–C=O–OH), and a side-chain (usually denoted as R) (Fig.1). They are required for the structure, function, and regulation of the body’s cells, tissues, and organs.
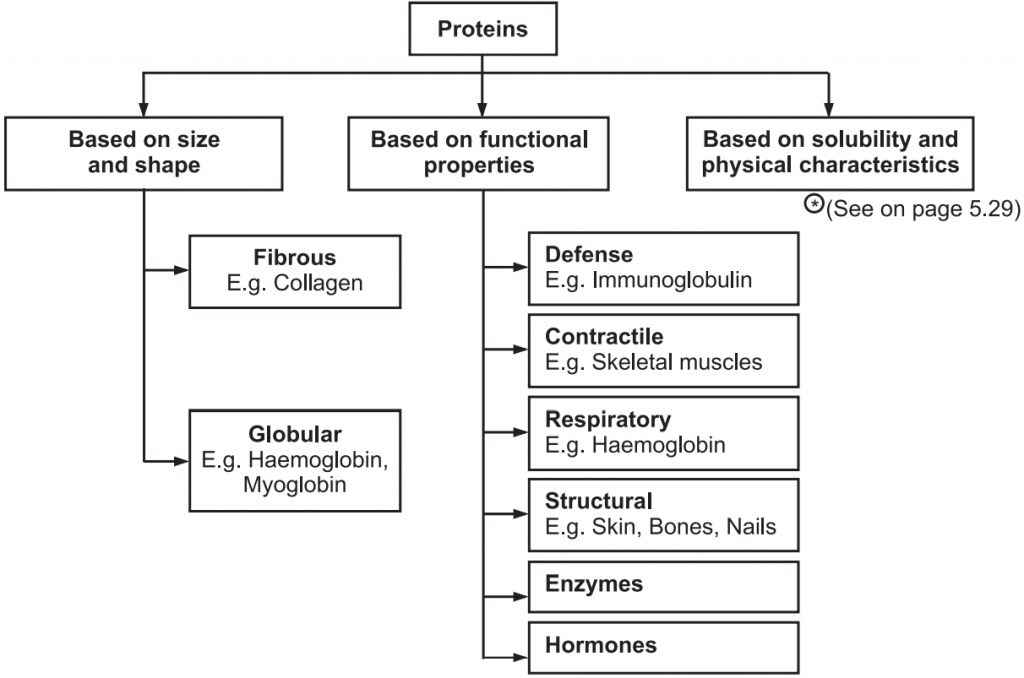
Examples: Hormones, enzymes
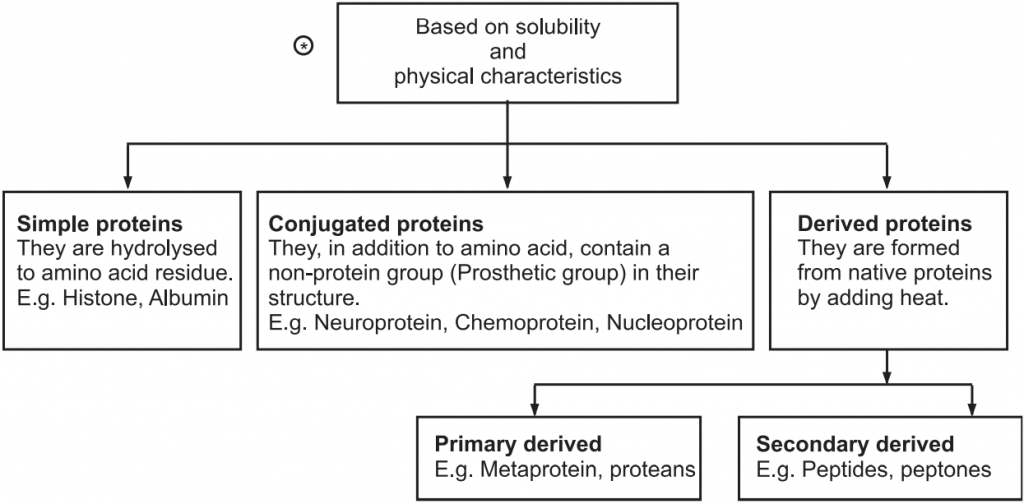
They are stored in the form of aleurone grains in plants and can be extracted easily. They are purified from other cellular components using a variety of techniques such as ultracentrifugation, precipitation, electrophoresis, and chromatography techniques.
Chemistry:
- The length of proteins and complexity vary based on the number and type of amino acid chains. There are about 20 different amino acids, each with a different chemical structure and characteristics; for instance, some are polar, others are non-polar. The final protein structure is dependent upon the composition of amino acids.
- They consist of two polypeptide chains, a long chain which is on the left side, consisting of 346 amino acids, and a short-chain which is on the right side having 99 amino acids. The long-chain is also known as a heavy chain that contains 5 domains. In that 3 are extracellular domains (N1, C1 and C2) and a transmembrane domain where the polypeptide chain passes through the cell and cytoplasmic domains (C-terminal) within the cytoplasm of the cell.
- They are hydrolyzed with acids or enzymes and break into amino acids.
- They form colloidal solutions in water.
- Proteins are amphoteric and get easily denatured due to heat, changes in pH, reaction with organic solvents etc.
Biochemical Importance:
- Proteins are the main structural and functional component of the cytoskeleton. They are the main source of replacement of nitrogen in the body.
- Proteins act as biocatalysts. They are enzymes.
- Proteins are immunoglobulins that serve as a first-line defence against bacteria.
- Structural proteins provide mechanical strength to the body.
- Storage proteins bind with specific substances and are stored in the body. For example, iron is stored in the body as ferritin.
- Transport proteins carry out the function of transporting specific substances either across the membrane or in the body fluids.
Classification of Proteins:
Table of Contents
Broadly proteins are classified into three types tabulated in Fig.2.
Isolation of Proteins:
Various modern isolation techniques are used for the isolation of proteins that include cell disruption method, mechanical homogenization, solid-phase micro-extraction, supercritical-fluid extraction, pressurized-liquid extraction, microwave-assisted extraction, solid-phase extraction, and surfactant-mediated techniques.
Generally, protein is extracted with trichloroacetic acid (TCA) and acetone or by using phenol or by homogenization with buffer. Plant tissue is homogenized in 10% TCA containing 2% beta-mercaptoethanol using liquid nitrogen. Further, the solution is kept at –20°C for overnight to form the precipitate which is subjected to centrifugation at high r.p.m for 30-40 minutes at 4°C. The precipitate is washed with cold acetone.
Analysis Methods of Proteins:
- Biuret Method: A violet-purplish colour is produced when cupric ions (Cu2+) interact with peptide bonds under alkaline conditions. The biuret reagent is mixed with a protein solution and then allowed to stand for 15-30 minutes and then the absorbance is taken at 540 nm.
- Lowry Method: The lowry method combines the biuret reagent with another reagent i.e. Folin-Ciocalteau phenol reagent which reacts with tyrosine and tryptophan residues in proteins. This gives a bluish colour which can be read somewhere between 500-750 nm. There is a small peak around 500 nm that can be used to determine high protein concentrations and a large peak around 750 nm that can be used to determine low protein concentrations.
- Turbimetric Method: Protein molecules that are normally soluble in solution are precipitated by the addition of certain chemicals, e.g., trichloroacetic acid. Protein precipitation causes the solution to become turbid and the final concentration of protein is determined by measuring the degree of turbidity.
Some other methods are like Kjeldahl method, Dye binding method, UV spectroscopy method etc.
General Chemical Tests for Proteins:
- Biuret Reaction: Sample solution is mixed with 10% sodium hydroxide and 0.1% copper sulphate solution. The solution becomes violet or pink colour. Compounds with two or more peptide bonds give a violet colour with alkaline copper sulphate solution. Proteins in the alkaline environment reduce Cu2+ to Cu+, which forms a coordination complex with proteins, leading to a blue to light violet colour change.
- Ninhydrin Test: Sample solution is mixed with 0.1% freshly prepared Ninhydrin solution and then boiled to get violet or purple colour.
- Xanthoproteic Reaction: Sample solution is mixed with a few ml of concentrated nitric acid and boiled. Then 40% sodium hydroxide is added slowly. The yellow colour of the solution turns to deep orange colour. The yellow colour is due to the nitro derivatives of the aromatic amino acids present in the protein. The sodium salts of nitro derivatives are orange in colour.
- Sulphur Test: Sample solution is mixed with a few ml of 40% NaOH and few drops of 2% lead acetate solution and then boiled. The solution forms a black precipitate after cooling.
- Sakaguchi Reaction: Sample solution is mixed with 0.02% alpha naphthol solution, 10% sodium hydroxide solution and few drops of alkaline hypobromite solution. The solution gives intense red colour.
Protein Related Drugs
Gelatin
Synonyms: Collagen Hydrolysate, Denatured Collagen, Gelatina.
Source: Gelatin is mainly polypeptide with higher molecular weight protein obtained by boiling skin, tendons, ligaments, and/or bones with water. It is usually obtained from cattle bones, cattle hides and pork skins.
Biological Source: Cattle: Bos taurus, Family: Bovinae Pork: Sus scrofa, Family: Suidae
Preparation: Gelatin is prepared by hydrolysis of collage. Animal skins and bones are used as raw materials. There are two main types of gelatin. Type-A gelatin has an isoionic point of 7-9. It is derived from collagen with exclusively acid pretreatment and it takes about 7-10 days. Whereas, Type-B gelatin has an isoionic point of 4.8 to 5.2. It is prepared by an alkaline pretreatment of the collagen and it takes more time than the former one. The bones are defatted and then decalcified with organic solvent and acid respectively to give a soft sponge-like material called ossein. Calcium phosphate is produced as a byproduct. The ossein is soaked in lime pits for several weeks for hydrolysis and then treated with hot water at 85°C to get solubilized gelatin from collagen. The resultant weak solution of gelatin is concentrated in a series of evaporators and chilled to form a gel. Gels are spread into metal trays and allowed to set into jelly and then removed by drying at 10°, 30° and 60°C for a month. They are further bleached in sulphur dioxide to produce light coloured gelatin, which is then dried at room temperature (Fig.3).
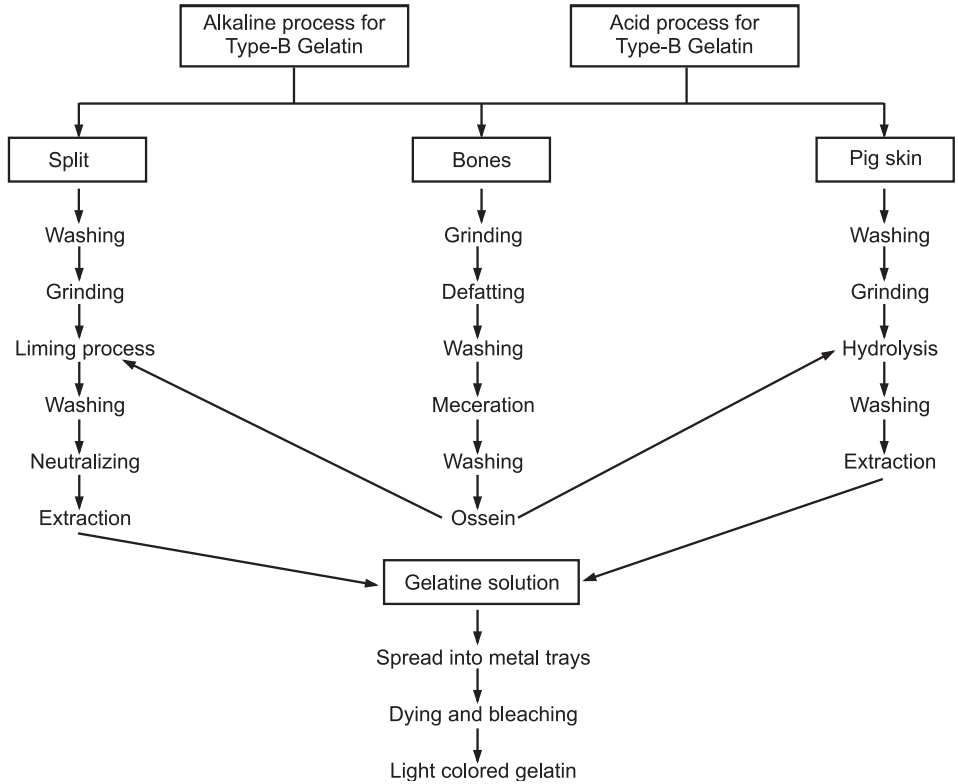
Physical Properties:
- Appearance: Translucent, brittle when dried.
- Colour: Colourless
- Odour: None
- Taste: Unpleasant
- Solubility: Soluble in hot water, but forms gel when cooled. Insoluble in alcohol, ether
- Stability: Stable in air, but in moisture conditions, it is degraded.
- Extra feature: In cold water, it swells and softens
- Strength: Determined by bloom strength (Bloom is a test to measure the strength of gelatin. The test was originally developed and patented in 1925 by O.T. Bloom. The test determines the weight in gm, needed by a probe with a diameter of 0.5 inches to deflect the surface of the gel 4 mm without breaking it. The result is expressed in Bloom. It is usually between 30 and 300 Bloom).
- Relative density: 1.3 to 1.4
- Isoionic point: 5-9
- Foaming property: Efficient foam stabilizer pH (1%) : 5
- Moisture: 16%
Chemical Constituents: Gelatin contains 98-99% protein by dry weight. Gelatin is unusually high in the non-essential amino acids like glycine (26%), proline (16%), hydroxyproline (14%), glutamic acid, alanine, arginine, aspartic acid, lysine, serine, byline, etc.
Chemical Tests:
- Aqueous sample solution + Tannic acid = White precipitation
- Aqueous sample solution + Picric acid = Yellow precipitation
- Aqueous sample solution + Millon’s reagent = White precipitation
- Aqueous sample solution + Soda-lime → Ammonia gas evolves
Sterile Gelatin: They are of two types like absorbable gelatin sponge and absorbable gelatin film. Sterile gelatin sponges are sterile white products. They are absorbable and water-insoluble. They are prepared by warming gelatin solution to form foam and then dried and cut into small pieces. They are finally sterilized at 150°C, whereas absorbable gelatin film is light amber coloured and transparent. They are insoluble in water.
Uses: It is used in face masks, shampoos and other cosmetics; as a thickener for fruit gelatins and puddings, in candies, marshmallows, cakes, ice cream, and yoghurts. It is used in the preparation of bacteriological culture media. It is also used on photographic films, in vitamins as a coating and as capsules shells. Medically it is used for weight loss, treating osteoarthritis, rheumatoid arthritis and brittle bones (osteoporosis).
Casein
Casein is related to phosphoprotein. These proteins are commonly found in mammalian milk, making up 80% of the proteins in cow milk and between 20% and 45% of the proteins in human milk.
Isolation:
- A specified amount of milk is kept in the flask and heated at 40°C in a water bath.
- Few drops of glacial acetic acid are added and stirred.
- The resultant mixture is filtered through filter paper held in a funnel and most of the liquid is gently squeezed out.
- Casein and fat are removed from the cheesecloth, the solid is placed into a beaker and a few ml of 95% ethanol is added.
- Then it is stirred well to break up the product. The liquid is poured off and a few ml of 1 : 1 ether-ethanol mixture is added to the solid.
- It is stirred well and filtered through filter paper.
- Solid is scraped into a weighed filter paper and then dried in the air.
- The casein content is then calculated as follows:
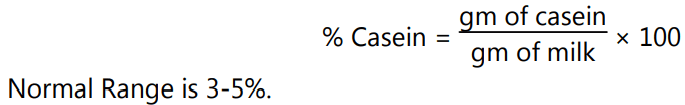
Properties:
- It is purified powder and yellow.
- It is found in milk as a suspension of particles called “casein micelles”.
- It is relatively hydrophobic.
- It is poorly soluble in water and insoluble in a neutral salt solution.
- The caseins in the micelles are held together by calcium ions and hydrophobic interactions.
- The isoelectric point of casein is 4.6.
- It is readily dispersible in dilute alkalis and salt solutions such as sodium oxalate and sodium acetate.
- The melting point is 280°C.
Chemical Nature:
- It is a phosphoprotein, which has phosphate groups attached to some of the amino acid side chains. Mostly these amino acids are serine and threonine.
- Casein is made up of the main 3 types of proteins − alpha-casein, beta-casein and kappa-casein.
- All casein proteins have different hydrophobic and hydrophilic regions along the protein chain.
- Alpha-caseins are the major casein proteins. They contain 8-10 phosphate groups.
- Beta-casein contains about 5 phosphate residues.
- Beta-casein is more hydrophobic than alpha-caseins and kappa-casein.
- Casein micelles consist of water, protein and salts.
- Casein is present as a caseinate that binds primarily calcium and magnesium.
Uses:
Casein is the major component of cheese. It is used as a food additive, binder for safety matches. As a food source, casein supplies amino acids, carbohydrates and the two inorganic elements calcium and phosphorus. Derivatives of Casein are used in tooth remineralization products to stabilize amorphous calcium phosphate. Casein peptides are used for high blood pressure, high cholesterol, anxiety, fatigue, epilepsy, intestinal disorders, cancer prevention and stress reduction.
Make sure you also check our other amazing Article on : Tragacanth Gum
